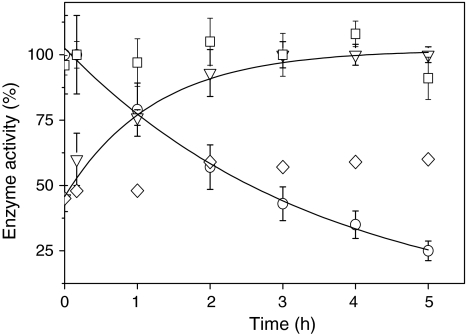
Fig. 7.
Effect of light on the activity of ascorbate peroxidase and on the activity of NADP-malate dehydrogenase (MDH) in vitro. Cells were incubated in the presence of 2 mM H2O2 in the light (white light, 70 μmol photons m−2 s−1) for the time indicated. H2O2 and DCMU were added at time point zero. Cells were harvested at the time indicated and opened up by freeze/thawing. Ascorbate peroxidase (squares) activity was measured photometrically at 290 nm in the crude extract in the presence of ascorbate and H2O2 as outlined in Sect. “Materials and methods”. 100% activity corresponds to an activity of 18 μmol dehydroascorbate mg protein−1 min−1 produced when 1 mM H2O2 was added as substrate. The activity of MDH was measured photometrically at 340 nm in the presence of NADPH and oxalacetate in the absence (inverted triangles) and in the presence of 6 μM DCMU (diamonds). Maximal activity (100%) was achieved by incubation of samples with DTT and thioredoxin h (see Sect. “Materials and methods” for details”). For comparison the catalase activity (circles) is shown (data taken from Fig. 6)