Abstract
Estimating the potential health benefits and expenditures of a partially effective HIV vaccine is an important consideration in the debate about whether HIV vaccine research should continue. We developed an epidemic model to estimate HIV prevalence, new infections, and the cost-effectiveness of vaccination strategies in the U.S. Vaccines with modest efficacy could prevent 300,000–700,000 HIV infections and save $30 billion in healthcare expenditures over 20 years. Targeted vaccination of high-risk individuals is economically efficient, but difficulty in reaching these groups may mitigate these benefits. Universal vaccination is cost-effective for vaccines with 50%-efficacy and price similar to other infectious disease vaccines.
Keywords: HIV vaccine, HIV prevention, Cost-effectiveness analysis
1. Introduction
The recent failure of a candidate HIV vaccine developed by Merck in a phase III trial has prompted calls for a fundamental reevaluation of investments in HIV vaccine research [1, 2]. This trial was the second failure in phase III, following the disappointing results from the AIDSVAX vaccine developed by VaxGen [3, 4]. These setbacks prompted the National Institutes of Allergy and Infectious Diseases (NIAID) to hold a conference with leaders in vaccine development to discuss research priorities [5]. Some advocacy groups called for a cessation of all funding for HIV vaccine development [6], while a number of investigators called for a termination of clinical trials and a shift to emphasize basic science research [1].
Investments in HIV vaccine research and development have increased almost three-fold in the past six years [7, 8], and more than 30 candidate vaccines are currently being evaluated in clinical trials [9]. However, funding was relatively modest at $760 million in 2006 given that 2.7 million people worldwide became newly infected with HIV in 2007, including nearly 60,000 new infections in the United States [10]. The challenges to successful vaccine development are formidable, and include the striking diversity of HIV subtypes, with circulating recombinant forms, rapid and ongoing viral evolution among individuals and populations, and mutational escape from immune control [11].
In the ongoing public debate about how and whether vaccine research should proceed, an important consideration is the potential health and economic benefit that could accrue from successful development of an HIV vaccine. If the potential health and economic benefits of a vaccine are large, then relatively large public investments in research may be warranted. Prior analyses (e.g., [12–36]) have evaluated the effect of vaccines in different settings, but no studies have comprehensively evaluated the potential population health benefits and costs across all risk groups in the United States.
To inform this debate, we evaluated the potential population health benefits and expenditures of alternative HIV vaccination strategies in the United States. We assessed outcomes for a broad range of vaccine efficacy and costs, and also assessed the outcomes associated with either universal vaccination, or vaccination targeted to high-risk groups. Our study is the first to evaluate both health and economic outcomes associated with universal or targeted vaccination in the U.S., and provides insight into the magnitude of the benefit a preventive HIV vaccine could provide.
2. Methods
We developed a dynamic compartmental model of HIV transmission and progression. The model is specified by a set of differential equations (Appendix). Using data from the U.S. (Table 1), we instantiated the model to simulate the HIV epidemic over a 20-year time horizon under different preventive vaccination scenarios. We estimated all healthcare costs incurred and benefits experienced in the population (measured as quality-adjusted life years (QALYs)), as well as HIV prevalence and new infections. Following standard practice, we discounted costs and QALYs to the present at 3% annually [37]. We calculated cost-effectiveness ratios for a variety of vaccination strategies. We conducted sensitivity analysis on all key model parameters. We implemented the model using the mathematical programming language Matlab.
Table 1.
Model parameters and sources
| Parameter | Value | Range | Source |
|---|---|---|---|
| Demographic Parameters | |||
| Total adult population | 154,141,198 | 150–160 million | [53] |
| Male IDU | 1,000,000 | 0.8–1.5 million | Calculated [7, 39, 52–55] |
| Male MSM | 4,662,913 | 3.5–5.5 million | Calculated [7, 39, 52, 53] |
| Male IDU/MSM | 300,000 | 200,000–500,000 | Calculated [7, 39, 52–57] |
| Male Other | 71,752,311 | 70–72 million | Calculated [7, 39, 52, 53] |
| Female IDU | 450,000 | 300,00–600,000 | Calculated [7, 39, 52–55] |
| Female Other | 75,975,974 | 75–77 million | Calculated [7, 39, 52, 53] |
| HIV prevalence | 0.7% | 0.6–1.0% | Calculated [7, 39, 52–55] |
| Male IDU | 13.6% | 10–20% | Calculated [7, 39, 52–55] |
| Male MSM | 10.6% | 5–20% | Calculated [7, 39, 52, 53] |
| Male IDU/MSM | 18.7% | 15–30% | Calculated [7, 39, 52–57] |
| Male Other | 0.14% | 0.05–0.25% | Calculated [7, 39, 52, 53] |
| Female IDU | 17.3% | 15–30% | Calculated [7, 39, 52–55] |
| Female Other | 0.29% | 0.15–0.40% | Calculated [7, 39, 52, 53] |
| Mortality rate a | |||
| Male | 0.0021 | 0.001–0.003 | Calculated [75] |
| Female | 0.0011 | 0.001–0.003 | Calculated [75] |
| Injection Drug User | 0.025 | 0–0.05 | [58] |
| Maturation rate b | |||
| Male | 0.0277 | 0.01–0.03 | Calculated [53] |
| Female | 0.0288 | 0.01–0.03 | Calculated [53] |
| Entry rate c | |||
| Male | 0.034 | 0.02–0.05 | Calculated [53] |
| Female | 0.033 | 0.02–0.05 | Calculated [53] |
| Disease Parameters | |||
| Quality-of-life factor | |||
| Uninfected | 1.0 | --- | [40] |
| Asymptomatic HIV – Untreated | 0.89 | 0.85–0.95 | [40, 76–79] |
| Symptomatic HIV – Untreated | 0.72 | 0.70–0.80 | [40, 76–79] |
| Symptomatic HIV – Treated with HAART | 0.83 | 0.82–0.87 | [40, 76–79] |
| AIDS – Untreated | 0.72 | 0.60–0.75 | [40, 76–79] |
| AIDS – Treated with HAART | 0.82 | 0.82–0.87 | [40, 76–79] |
| Injection Drug User (multiplier) d | 0.9 | 0.80–1.0 | [48, 58] |
| Injection Drug Use Parameters | |||
| Transmission probability per shared injection | |||
| Asymptomatic HIV | 0.002 | 0.001–0.005 | [48, 58] |
| Symptomatic HIV | 0.003 | 0.001–0.005 | [48, 58] |
| AIDS | 0.003 | 0.001–0.005 | [48, 58] |
| Average injections per year | 200 | 100–500 | [48, 58, 72] |
| Fraction of injections that are shared | 20% | 10–40% | [58, 61, 71, 73] |
| Sexual Behavior Parameters | |||
| Annual transmission probability per partnership | |||
| Heterosexual (FHIV+ → MHIV−) | |||
| Asymptomatic HIV | 0.020 | 0.010–0.040 | [40] |
| Symptomatic HIV | 0.026 | 0.010–0.040 | [40] |
| AIDS | 0.065 | 0.030–0.060 | [40] |
| Heterosexual (MHIV+ → FHIV−) | |||
| Asymptomatic HIV | 0.030 | 0.020–0.050 | [40] |
| Symptomatic HIV | 0.040 | 0.020–0.050 | [40] |
| AIDS | 0.100 | 0.050–0.090 | [40] |
| Homosexual (MHIV+ → MHIV−) | |||
| Asymptomatic HIV | 0.040 | 0.030–0.060 | [40] |
| Symptomatic HIV | 0.050 | 0.030–0.060 | [40] |
| AIDS | 0.150 | 0.080–0.120 | [40] |
| Annual number of same-sex partners | |||
| Male MSM | 3.0 | 2.0–5.0 | [62–64] |
| Male IDU/MSM | 3.0 | 2.0–5.0 | [61–63] |
| Condom usage with same-sex partners | |||
| Male MSM | 40% | 30–60% | [56, 62–64] |
| Male IDU/MSM | 40% | 30–50% | [61] |
| Annual number of opposite-sex partners | |||
| Male IDU | 3.0 | 2.0–5.0 | [65] |
| Male MSM | 0.1 | 0–1.0 | [64] |
| Male IDU/MSM | 0.1 | 0–1.0 | [66] |
| Male Other | 1.1 | 0.5–2.0 | [64, 67–70] |
| Female IDU | 3.5 | 2.0–5.0 | [65] |
| Female Other | 1.1 | 0.5–2.0 | [67–70] |
| Condom usage with opposite-sex partners | |||
| Male IDU | 25% | 15–35% | [56, 66] |
| Male MSM | 30% | 20–50% | [56, 61] |
| Male IDU/MSM | 30% | 30–50% | [61, 66] |
| Male Other | 20% | 10–40% | [67] |
| Female IDU | 25% | 20–50% | [65, 71] |
| Female Other | 20% | 10–40% | [67] |
| Treatment Parameters | |||
| Fraction starting HAART at symptom onset | 50% | 25–75% | Estimated [40] |
| HAART initiation rate after symptom onset | 0.05 | 0–0.10 | Estimated [40] |
| Reduction in injection infectivity due to HAART | 50% | 25–75% | [40, 48] |
| Reduction in sexual infectivity due to HAART | 90% | 50–99% | [40, 44–46, 48–51] |
| Circumcision Parameters | |||
| Fraction of males circumcised | 70% | 50–80% | [80] |
| Reduction in HIV acquisition due to circumcision | 50% | 48–60% | [59, 60] |
| Cost Parameters | |||
| Annual HIV-related healthcare costs | |||
| Asymptomatic HIV – Untreated | $3,967 | $3,000–$6,000 | [81, 82] |
| Symptomatic HIV – Untreated | $6,660 | $5,000–$9,000 | [81, 82] |
| Symptomatic HIV – Treated with HAART | $5,937 | $5,000–7,000 | [81, 82] |
| AIDS – Untreated | $21,000 | $15,000–$25,000 | [81–84] |
| AIDS – Treated with HAART | $9,557 | $6,000–$17,000 | [40, 82] |
| Annual non-HIV-related healthcare costs | $6,728 | $5,000–$8,000 | [85] |
| Annual cost of HAART | $14,974 | $12,000–$18,000 | [40, 82, 84] |
| Annual cost of IDU services | $2,500 | $1,000–$4,000 | [58] |
| Annual discount rate | 3% | 0–5% | [37] |
IDU = Injection drug user, MSM = Men who have sex with men, Other = General population.
HAART = Highly active antiretroviral therapy.
Mortality rate = non-HIV-related mortality rate among the population aged 15 to 49 years.
Maturation rate = rate 49-year olds turn age 50 and exit the population.
Entry rate = rate 14-year olds turn age 15 and enter the population.
Quality-of-life for all injection drug users is multiplied by this quantity.
2.1. Population Groups
We subdivided the adult population aged 15 to 49 into 144 compartments, based on gender, risk behavior, and infection, treatment, and vaccination status. An estimated 1.1 million adults were living with HIV in the U.S. in 2007, including approximately 300,000 women [7]. We subdivided men into four risk groups: injection drug users (IDUs), men who have sex with men (MSM), IDU/MSM, and the general population. Men in the IDU, MSM, and IDU/MSM compartments represent high-risk groups, and account for approximately 17%, 62%, and 7% of HIV-infected men living in the U.S., respectively [10, 38, 39]. Men in the general population are low-risk individuals and account for 13% of HIV cases among men [39]. We subdivided women into two groups: IDUs and the general population. Female IDUs are high-risk individuals who account for 26% of HIV cases among women in the U.S. Women in the general population are low-risk individuals and account for 73% of cases [39].
We stratified each risk group based on HIV infection status: asymptomatic HIV, symptomatic HIV, and AIDS. We subdivided the HIV-infected population based on treatment status, if eligible. We subdivided male groups based on circumcision status, and subdivided all groups based on preventive vaccine status. Stratifying the population along these dimensions allowed us to capture differences in the likelihood of acquiring or transmitting HIV, and potential variations in risk behaviors.
2.2. Disease Progression and Treatment
We estimated the average duration of each disease stage (asymptomatic HIV, symptomatic HIV, and AIDS), both in the absence and presence of highly active antiretroviral therapy (HAART), based on data from a published Markov model of the natural history of HIV [40]. Individuals progress to subsequent disease stages or death at rates inversely proportional to the average time spent in the current disease stage.
Because we wanted to examine the impact of a vaccine program, rather than a screening program, we assumed that all individuals are aware of their HIV status. We assumed that individuals with symptomatic HIV and AIDS are eligible to receive HAART, consistent with current U.S. treatment guidelines [40–43]. Currently, not all individuals who are identified with HIV are receiving HAART. We assumed that 50% of eligible HIV-infected individuals initiated HAART upon developing symptomatic HIV, with 5% of the remaining untreated population entering treatment annually [40].
Suppressive HAART reduces an infected individual’s viral load, which reduces disease progression and mortality. A reduced viral load is also thought to lower an individual’s infectivity, thereby reducing the probability of HIV transmission [40, 44–51]. However, infected individuals who receive HAART and live longer can engage in risky sexual and needle-sharing behaviors during their increased lifespan, thus potentially increasing HIV transmission. Our dynamic model can quantify the effects on the epidemic of these opposing forces.
2.3. Vaccination
We defined a preventive vaccine as one that confers partial or full immunity in uninfected, vaccinated individuals. We assumed that a preventive vaccine has an average duration of effectiveness, which we varied from one year to lifelong protection; after this time, individuals transition back to an unvaccinated state. Vaccinated individuals have a lower chance of acquiring HIV, which we defined as the vaccine efficacy. We also considered the possibility of behavior change due to vaccination, and we varied the number of sexual partners to reflect possible changes in risk behavior.
We evaluated the effects and cost-effectiveness of various preventive vaccination strategies, including universal vaccination (all groups), and vaccination targeted to high-risk (IDU, MSM, and IDU/MSM) or low-risk (general population) groups. We assumed that some fraction of unvaccinated individuals is initially vaccinated at time zero; we refer to this initial fraction as the vaccine coverage.
2.4. HIV Transmission
The model includes sexual transmission (male-to-female, female-to-male, and male-to-male) and from needle-sharing during injection drug use. We modeled infection transmission using binomial processes and assumed proportional mixing in the population (Appendix). We calculated the probability of sexual transmission on a per partnership basis, and calculated the probability of needle-sharing transmission per shared needle between an uninfected IDU and infected IDU. We adjusted the transmission probability to account for the infected individual’s gender, disease state, and treatment status, and the uninfected individual’s gender, circumcision status (if male), and vaccination status. We assumed the fraction of men circumcised remains at current levels.
2.5. Model Parameters
We estimated values for all model parameters based on published literature and expert opinion (Table 1). Because many behavioral and biological parameters are uncertain (e.g., number of sexual partnerships, probability of HIV transmission per partnership), we varied all parameters in sensitivity analysis.
We considered the entire adult population of the United States. We estimated risk group sizes and initial HIV prevalence levels based on available data. We estimated initial HIV prevalence to be 14% in male IDU, 11% in male MSM, 19% in male IDU/MSM, 0.14% in heterosexual males, 17% in female IDU, and 0.29% in heterosexual females [7, 10, 38, 39, 52–57]. We estimated annual entry, maturation, and mortality rates for each risk group based on available demographic data.
We estimated relevant biological parameters, including the probability of HIV transmission per sexual partnership or shared needle [48, 58], the reduction in HIV acquisition due to male circumcision [59, 60], and the reduction in sexual or needle-sharing infectivity due to HAART [40, 44–46, 48–51]. We also estimated behavioral parameters, including the annual number of same-sex and opposite-sex partners [61–70], condom use [56, 61–67, 71], annual number of drug injections [48, 58, 72], and needle-sharing rates [58, 61, 71, 73].
Finally, we estimated quality-of-life adjustments and all healthcare costs for each health state, and we considered a range of vaccine costs. All healthcare costs are given in 2007 U.S. dollars.
We validated our model by comparing the model-estimated prevalence to published estimates of HIV prevalence and incidence for each risk group over the past five years. Our model’s estimates of HIV prevalence were very similar to observed trends in prevalence among the general population. Estimates of prevalence among high-risk groups are more uncertain; however, our model’s projected prevalence reasonably approximated available data.
3. Results
3.1. Health Outcomes
3.1.1. HIV Infections Prevented
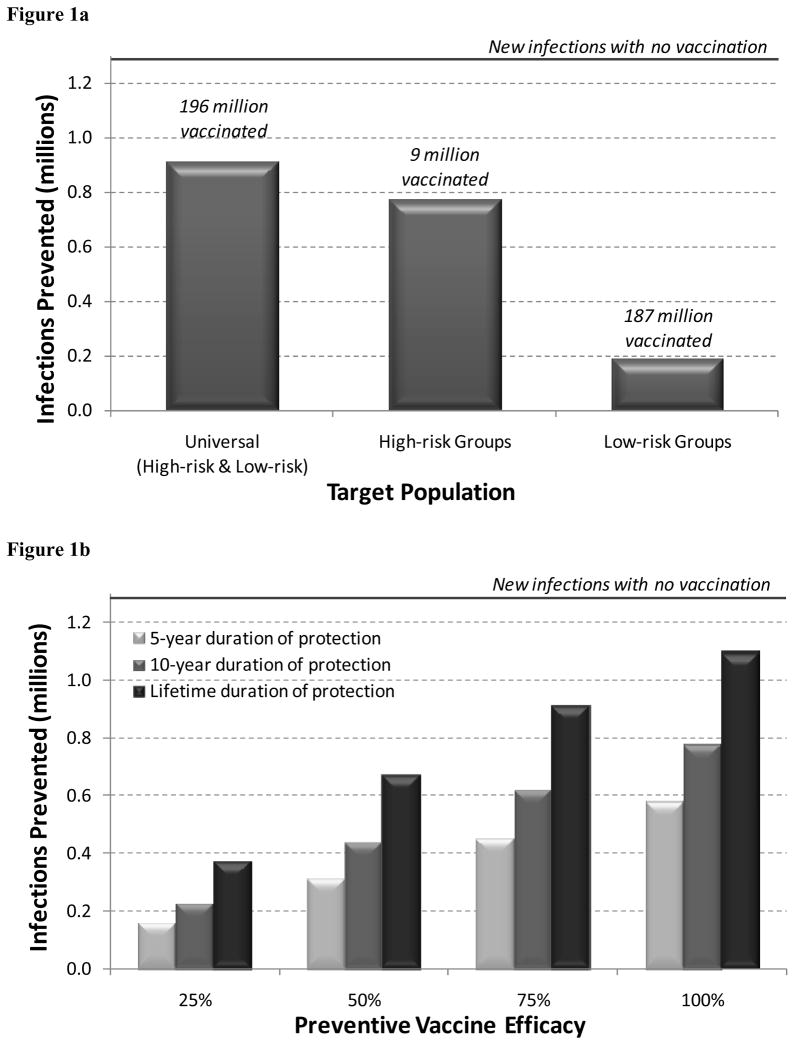
With no HIV vaccination program, we estimated that 1.29 million new HIV infections would occur over 20 years. A vaccination program targeting 75% of uninfected high-risk individuals averted 774,000 HIV infections over 20 years (60% of projected new infections), assuming a vaccine with 75% efficacy and lifetime duration of protection (Figure 1a). With this strategy, approximately 9 million high-risk individuals were vaccinated. If the vaccination program instead reached 25%, 50%, or 100% of high-risk individuals, then 320,000, 573,000, or 933,000 HIV infections were prevented, respectively. In contrast, a vaccination program reaching the same fraction of low-risk individuals prevented 75% fewer infections, and required vaccination of 187 million people.
Figure 1.
HIV infections prevented over 20 years with different vaccination strategies.
(a) HIV infections prevented with universal, high-risk group targeted, or low-risk group targeted vaccination. Assumes a vaccine with 75% efficacy and lifetime duration of protection, and 75% coverage of the target population.
(b) HIV infections prevented under different vaccine efficacy (25%, 50%, 75%, 100%) and duration (5-year, 10-year, lifetime) scenarios. Assumes universal vaccination (both high-risk and low-risk groups) with 75% coverage.
Universal vaccination prevented the greatest number of HIV infections (912,000, or 71% of projected new infections), but required vaccinating the greatest number of people (196 million). With universal vaccination, an estimated 110,000 (12%) HIV infections prevented are among the unvaccinated population, due to reduced secondary transmission by vaccinated individuals.
Because the efficacy of an HIV vaccine is unknown, we evaluated vaccines with varying degrees of efficacy, from 25% to 100% (Figure 1b). A vaccine with only 50% efficacy (and lifetime duration of protection) prevented 673,000 infections, whereas a vaccine with 100% efficacy prevented 1.10 million infections, assuming universal vaccination with 75% coverage.
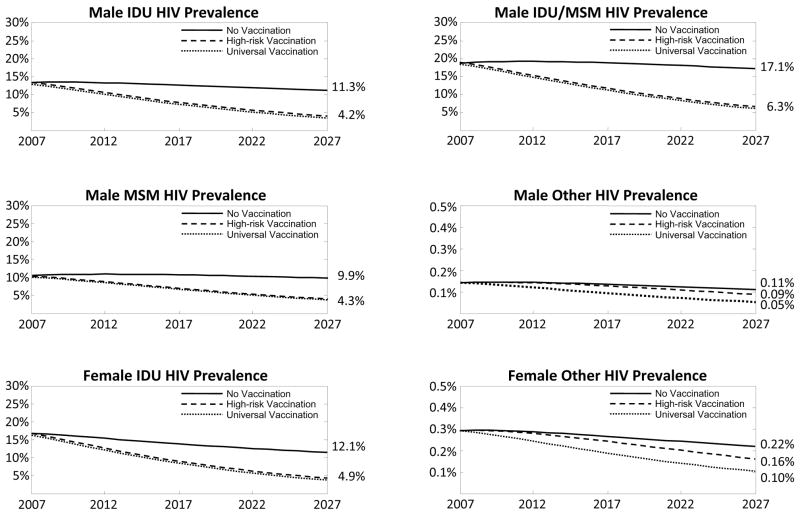
3.1.2. HIV Prevalence
Exclusively vaccinating high-risk groups not only reduced HIV prevalence among these individuals, but also substantially decreased prevalence among low-risk individuals, due to reduced secondary transmission (Figure 2). Vaccinating 75% of high-risk individuals reduced HIV prevalence among the unvaccinated general population from 0.11% to 0.09% among men, and from 0.22% to 0.16% among women after 20 years. Universal vaccination resulted in the lowest HIV prevalence in every group. After 20 years, HIV prevalence in the general population decreased to 0.05% among men and to 0.10% among women, a substantial improvement over exclusively vaccinating only high-risk or low-risk groups.
Figure 2.
HIV prevalence in the six population risk groups under universal and high-risk group targeted vaccination strategies. Assumes a vaccine with 75% efficacy and lifetime duration, and 75% coverage of the target population. IDU = Injection drug user, MSM = Men who have sex with men, Other = General population.
3.2. Economic Outcomes
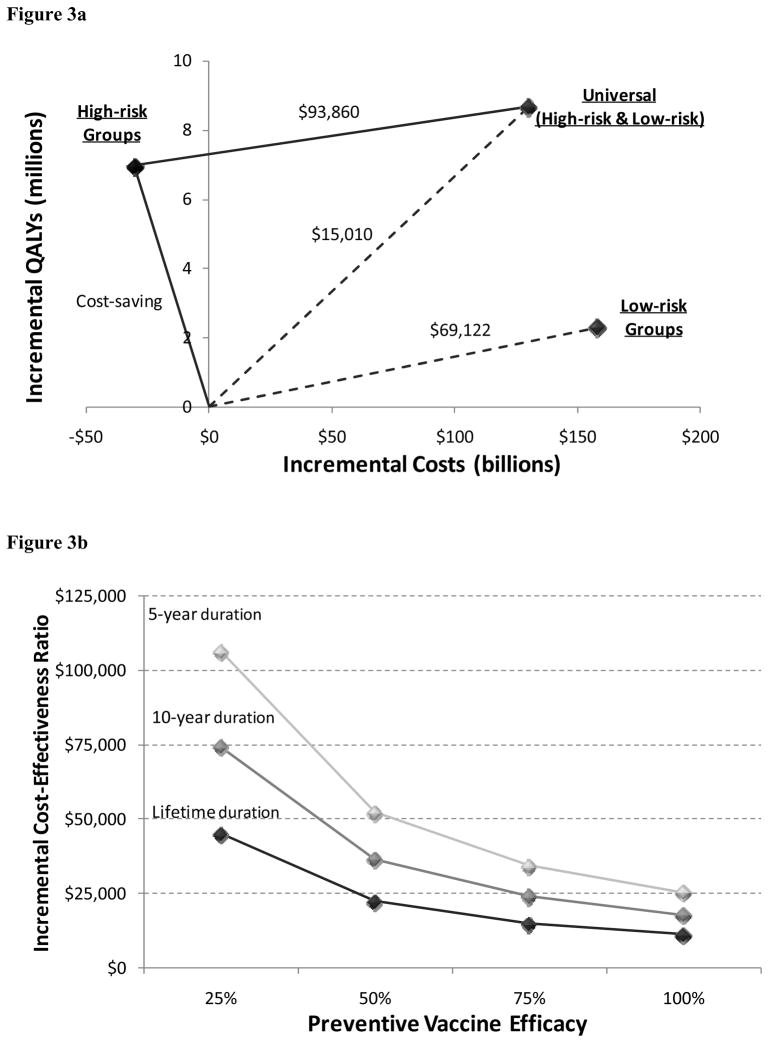
We evaluated the incremental cost-effectiveness of various preventive vaccination strategies. In the base case (a vaccine with 75% efficacy, lifetime duration, and $1000 price), strategies that exclusively vaccinated high-risk groups were cost-saving (i.e., increased QALYs and decreased costs) relative to the status quo. Over 20 years, a vaccination program would add 7.0 million (discounted) QALYs and save $31 billion (discounted).
The cost-effectiveness of universal vaccination depends on the comparison group. If high-risk group vaccination is infeasible due to difficulties in reaching such populations, then the appropriate comparison is universal vaccination versus no vaccination, which results in a cost-effectiveness ratio of $15,010 per QALY gained. If high-risk group vaccination is feasible, then universal vaccination cost $93,860 per QALY gained compared to high-risk vaccination, assuming 75% coverage (Table 2, Figure 3a).
Table 2.
Preventive vaccination results
| Vaccine Scenario | New HIV Infections | Incremental Costsb (billions) | Incremental QALYs b (millions) | ICER relative to: |
|
|---|---|---|---|---|---|
| Targeted Populationa | No vaccination | High-risk Vaccination | |||
| No Vaccination | 1,286,009 | --- | --- | --- | --- |
| 75% Efficacy, Lifetime Duration, Price $1000 | |||||
| Universal Vaccination | 373,757 | $130.2 | 8.7 | $15,010 | $93,860 |
| High-risk Group Vaccination | 512,044 | −$30.5 | 7.0 | Cost-saving | --- |
| 50% Efficacy, Lifetime Duration, Price $1000 | |||||
| Universal Vaccination | 613,121 | $142.3 | 6.3 | $22,435 | $126,416 |
| High-risk Group Vaccination | 718,739 | −$20.1 | 5.1 | Cost-saving | --- |
| 75% Efficacy, 5-year Duration, Price $1000 | |||||
| Universal Vaccination | 837,566 | $152.7 | 4.4 | $34,447 | $193,080 |
| High-risk Group Vaccination | 902,169 | −$11.7 | 3.6 | Cost-saving | --- |
| 75% Efficacy, Lifetime Duration, Price $500 | |||||
| Universal Vaccination | 373,757 | $42.5 | 8.7 | $4,893 | $42,599 |
| High-risk Group Vaccination | 512,044 | −$34.4 | 7.0 | Cost-saving | --- |
Universal Vaccination = Vaccination of high-risk and low-risk groups.
ICER = Incremental cost-effectiveness ratio, relative to no vaccination or high-risk group vaccination.
Efficacy = Decrease in vaccinated individual’s chance of acquiring HIV.
Duration = Average length of protection conferred by preventive vaccine.
All results in this table assume 75% vaccination coverage of the targeted population.
Incremental costs and quality-adjusted life years (QALYs) are relative to no vaccination.
Figure 3.
Cost-effectiveness analysis of HIV vaccination over 20 years
(a) Cost-effectiveness of universal, high-risk group targeted, or low-risk group targeted vaccination. Assumes a vaccine with 75% efficacy, lifetime duration of protection, and $1000 price, and 75% coverage of the target population.
(b) Cost-effectiveness of universal vaccination under different vaccine efficacy (25%, 50%, 75%, 100%) and duration of protection (5-year, 10-year, lifetime) scenarios. Assumes a vaccine with $1000 price, and 75% coverage of the target population. Incremental cost-effectiveness ratios are relative to the status quo (no vaccination).
We evaluated the cost-effectiveness of vaccines with varying degrees of efficacy and duration (Table 2, Figure 3b). Universal vaccination with a 50%-efficacy vaccine cost $22,435 to $52,400 per QALY gained relative to no vaccination (and $126,416 to $279,071 per QALY gained compared to high-risk group vaccination), depending on the duration of protection. A vaccine with 100% efficacy cost $11,402 to $25,518 per QALY gained relative to no vaccination (and $78,176 to $150,318 per QALY gained compared to high-risk group vaccination).
Finally, we considered variations in vaccine price (Table 2). At a vaccine price of $500, universal vaccination with a 75% effective, lifetime-duration vaccine cost $4,893 per QALY gained relative to no vaccination (and $42,599 compared to high-risk group vaccination).
3.3. Sensitivity Analysis
3.3.1. Rate of New Infections
In the base case, our model estimated 1.29 million new infections would occur over 20 years. Although we validated our model estimates for the past five years by comparing the estimated prevalence from the model to available published estimates, there is uncertainty about HIV transmission rates over the next two decades. If the probability of HIV transmission (via sexual contact and needle-sharing) was 25% lower than we initially estimated, 808,000 infections occurred over 20 years. Under this scenario, universal vaccination cost $27,840 per QALY gained relative to no vaccination, assuming a 75%-efficacy, lifetime-duration vaccine. The general conclusions of our analysis remained unchanged, although the number of infections prevented and savings in expenditures were smaller.
3.3.2. Behavioral Change
The base case assumed no change in behavior due to preventive vaccination; however, the extent of potential behavioral disinhibition in response to HIV vaccination is uncertain. If vaccinated individuals had 25% more sexual partners than their unvaccinated counterparts, then vaccination programs prevented more HIV infections, for vaccines with at least 65% efficacy. This paradoxical finding is due to our assumption of proportional mixing within the population.
Vaccinated individuals who increased their number of sexual partners accounted for a larger fraction of the overall number of sexual partnerships in the population. An HIV-infected individual had a higher chance of randomly selecting a vaccinated partner (who was partially protected from acquiring HIV), which reduced the overall number of new HIV infections. However, the reverse effect occurred (i.e., vaccination programs averted fewer infections) at vaccine efficacy levels less than 65%. The protection conferred through vaccination was not enough to offset the increase in risky sexual behavior. At any efficacy level, the relative ranking of vaccination strategies remained unchanged.
3.3.3. HAART Effectiveness
We assumed HAART reduced the probability of HIV transmission via sexual contact and needle-sharing by 90% and 50%, respectively. If HAART was less effective in reducing sexual (50%) and needle-sharing (25%) transmission, all vaccination strategies averted more infections and were more cost-effective than in the base case. Because a preventive vaccine and HAART both reduce HIV transmission, they act as partial substitutes for each other. When the benefits offered by HAART decreased, the relative benefits of a preventive vaccine increased.
4. Discussion
Our analysis indicates that a successful HIV vaccine would provide enormous health and economic benefits. A highly effective vaccine could reduce healthcare expenditures by up to $40 billion over a 20-year period if targeted to high-risk groups in the United States. A fully protective vaccine used broadly in the population could prevent 1.10 million HIV infections over this period, adding 10.6 million QALYs to the population. Most importantly, a vaccine with only modest efficacy could provide significant benefit and good value. A vaccine that prevented infection in only 50% of recipients could prevent 310,000 to 673,000 infections over 20 years, with universal vaccination. Furthermore, approximately 12% of prevented HIV infections are among unvaccinated individuals, emphasizing the importance of reduced secondary transmission due to vaccination.
Targeted vaccination of high-risk groups is more economically efficient than universal vaccination, although universal vaccination provides the greatest total health benefit. A concern with targeted vaccination is that high-risk individuals may not self-identify, or may be unaware of risk behaviors, making it difficult to reach these groups. If high-risk vaccination is not feasible, the appropriate comparison for universal vaccination is no vaccination. Under these circumstances, universal vaccination meets conventional cost-effectiveness criteria with a vaccine price of $1000, and would be more economically efficient with less expensive vaccines. If high-risk group vaccination is feasible, universal vaccination is more expensive compared to high-risk vaccination.
Our findings are broadly consistent with prior studies evaluating the cost-effectiveness of HIV vaccination in a late-stage epidemic setting [12–15]. Although the cost-effectiveness of a partially effective HIV vaccine is more favorable in a high prevalence setting [15], our sensitivity analyses of variations in vaccine efficacy and duration of protection are consistent with prior modeling studies. Our finding that behavioral disinhibition in recipients of low-efficacy vaccines may attenuate the benefits associated with HIV vaccination is consistent with prior studies [36, 74]. However, we also find that increased sexual behavior among those vaccinated with high-efficacy vaccines can actually improve epidemic outcomes, because these individuals account for a greater proportion of sexual partnerships and offer indirect vaccine protection to their partners.
Estimating the health and economic benefits from a partially effective HIV vaccine is useful for understanding the potential return on investments in vaccine research. In this study, we evaluated only the benefits and expenditures in the U.S. If a vaccine were effective across subtypes of the virus and could be used worldwide, the health benefits would be many times higher. Our analysis suggests that each infection prevented saves approximately ten discounted quality-adjusted life years (and 16 undiscounted life years). The gain in life expectancy from HIV prevention may be different in developing countries because of shorter average life expectancy and lower average age of initial HIV infection. If a vaccine prevented half of the projected 40 million new infections worldwide over the next 20 years, an additional 200 to 300 million life years would accrue.
Our findings emphasize the importance of including high-risk individuals in any vaccination strategy to realize the potential health and economic benefits. Exclusively vaccinating only high-risk groups reduces HIV prevalence among these individuals, and also substantially reduces prevalence among low-risk groups. This additional benefit occurs because high-risk groups are key drivers of the HIV epidemic; vaccinating a portion of these individuals reduces secondary transmission to members of the general population. A universal vaccination strategy should be designed to ensure that high-risk individuals participate fully.
Our analysis has several limitations. We assumed proportional mixing within the population, which oversimplifies the complex network structure inherent in sexual and needle-sharing contacts. The model accounts for proportional mixing across risk groups (i.e., groups based on the number and type of sexual and needle-sharing contacts), but not across age stratifications. We included the adult population aged 15 to 49 because these individuals account for most new infections in the U.S. Including older individuals would minimally change our results. We assumed that all individuals were aware of their HIV status, to avoid confounding the effects of HIV screening with implementing a vaccination program. However, our modeling framework enables us to consider the additional effects of HIV screening, which would necessitate accounting for the additional cost of a universal screening program prior to vaccination.
Our present analysis considers vaccines aimed at preventing HIV acquisition in uninfected recipients. We recognize that the underlying vaccine mechanisms may influence whether the vaccine provides benefit to infected individuals via reduced disease progression. Our modeling framework could be extended to examine the benefits of such a vaccine on epidemic outcomes.
In summary, our analysis is the first to quantitatively estimate the potential health and economic outcomes of targeted or universal HIV vaccination programs in the U.S. A vaccination program that includes high-risk individuals could provide millions of life years of benefit over 20 years and meet conventional cost-effectiveness criteria. Partially effective vaccines, even with efficacy of only 50%, would provide substantial benefit at less cost than that of many interventions currently considered cost-effective. Investment in HIV vaccine research, although increasing in recent years, remains modest relative to the potential health and economic benefits of a successful vaccine.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse, United States National Institutes of Health (R-01-DA-15612) and the United States Department of Veterans Affairs. Each author reports no conflict of interest or any external financial support that is relevant to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaiser J. AIDS research. Review of vaccine failure prompts a return to basics. Science. 2008 Apr 4;320(5872):30–1. doi: 10.1126/science.320.5872.30. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J. AIDS research. Did Merck's failed HIV vaccine cause harm? Science. 2007 Nov 16;318(5853):1048–9. doi: 10.1126/science.318.5853.1048. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. Public health. AIDS vaccine trial produces disappointment and confusion. Science. 2003 Feb 28;299(5611):1290–1. doi: 10.1126/science.299.5611.1290. [DOI] [PubMed] [Google Scholar]
- 4.VaxGen. VaxGen Announces Initial Results of its Phase III AIDS Vaccine Trial. Brisbane, California: 2003. Feb 24, [Google Scholar]
- 5.NIAID to convene HIV vaccine summit. AIDS Patient Care STDS. 2008 Apr;22(4):350–1. [PubMed] [Google Scholar]
- 6.Brown D. Washington Post. 2008. AIDS Vaccine Testing at Crossroads. [Google Scholar]
- 7.Joint United Nations Programme on HIV/AIDS (UNAIDS). 2008 Report on the Global AIDS Epidemic; 2008.
- 8.Joint United Nations Programme on HIV/AIDS (UNAIDS). 2006 Report on the Global AIDS Epidemic; 2006.
- 9.International AIDS Vaccine Initiative (IAVI). Progress and Challenges. 2007 [cited September 18, 2008]; Available from: http://www.iavi.org/viewpage.cfm?aid=12
- 10.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008 Apr 10;358(15):1590–602. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards DM, Shachter RD, Owens DK. A dynamic HIV-transmission model for evaluating the costs and benefits of vaccine programs. Interfaces. 1998;28(3):144–66. [Google Scholar]
- 13.Owens DK, Edwards DM, Shachter RD. Population effects of preventive and therapeutic HIV vaccines in early- and late-stage epidemics. AIDS. 1998 Jun 18;12(9):1057–66. [PubMed] [Google Scholar]
- 14.Owens DK, Edwards DM, Shachter RD. Costs and benefits of imperfect HIV vaccines: Implications for vaccine development and use. In: Kaplan EH, Brookmeyer R, editors. Quantitative Evaluation of HIV Prevention Programs. Yale University Press; 2001. pp. 143–71. [Google Scholar]
- 15.Owens DK, Edwards DM, Cavallaro JF, Shachter RD. The cost effectiveness of partially effective HIV vaccines. In: Brandeau ML, Sainfort F, Pierskalla WP, editors. Operations Research and Health Care: A Handbook of Methods and Applications. Kluwer Academic Publishers; 2004. pp. 403–18. [Google Scholar]
- 16.Cowley P. Preliminary cost-effectiveness analysis of an AIDS vaccine in Abidjan, Ivory Coast. Health Policy. 1993 May;24(2):145–53. doi: 10.1016/0168-8510(93)90031-j. [DOI] [PubMed] [Google Scholar]
- 17.Edmunds WJ, Medley GF, Nokes DJ. Evaluating the cost-effectiveness of vaccination programmes: a dynamic perspective. Stat Med. 1999 Dec 15;18(23):3263–82. doi: 10.1002/(sici)1097-0258(19991215)18:23<3263::aid-sim315>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Halloran ME, Haber M, Longini IM., Jr Interpretation and estimation of vaccine efficacy under heterogeneity. Am J Epidemiol. 1992 Aug 1;136(3):328–43. doi: 10.1093/oxfordjournals.aje.a116498. [DOI] [PubMed] [Google Scholar]
- 19.McLean AR, Blower SM. Imperfect vaccines and herd immunity to HIV. Proc Biol Sci. 1993 Jul 22;253(1336):9–13. doi: 10.1098/rspb.1993.0075. [DOI] [PubMed] [Google Scholar]
- 20.Blower SM, McLean AR. Prophylactic vaccines, risk behavior change, and the probability of eradicating HIV in San Francisco. Science. 1994 Sep 2;265(5177):1451–4. doi: 10.1126/science.8073289. [DOI] [PubMed] [Google Scholar]
- 21.McLean AR, Blower SM. Modelling HIV vaccination. Trends Microbiol. 1995 Dec;3(12):458–62. doi: 10.1016/s0966-842x(00)89010-1. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RM, Garnett GP. Low-efficacy HIV vaccines: potential for community-based intervention programmes. Lancet. 1996 Oct 12;348(9033):1010–3. doi: 10.1016/s0140-6736(96)07100-0. [DOI] [PubMed] [Google Scholar]
- 23.Stover J, Garnett GP, Seitz S, Forsythe S. The World Bank. 2002. The epidemiological impact of an HIV/AIDS vaccine in developing countries. [Google Scholar]
- 24.Gray RH, Li X, Wawer MJ, Gange SJ, Serwadda D, Sewankambo NK, et al. Stochastic simulation of the impact of antiretroviral therapy and HIV vaccines on HIV transmission; Rakai, Uganda. AIDS. 2003 Sep 5;17(13):1941–51. doi: 10.1097/00002030-200309050-00013. [DOI] [PubMed] [Google Scholar]
- 25.Nagelkerke NJD, De Vlas SJ. The World Bank. 2003. The epidemiological impact of an HIV vaccine to the HIV/AIDS epidemic in Southern India. [Google Scholar]
- 26.Elbasha EH, Gumel AB. Theoretical assessment of public health impact of imperfect prophylactic HIV-1 vaccines with therapeutic benefits. Bull Math Biol. 2006 Apr;68(3):577–614. doi: 10.1007/s11538-005-9057-5. [DOI] [PubMed] [Google Scholar]
- 27.Anderson R, Hanson M. Potential public health impact of imperfect HIV type 1 vaccines. J Infect Dis. 2005 Feb 1;191(Suppl 1):S85–96. doi: 10.1086/425267. [DOI] [PubMed] [Google Scholar]
- 28.Stover J. Estimating the global impact of an AIDS vaccine. International AIDS Vaccine Initiative (IAVI); 2005. [Google Scholar]
- 29.Amirfar S, Hollenberg JP, Abdool Karim SS. Modeling the impact of a partially effective HIV vaccine on HIV infection and death among women and infants in South Africa. J Acquir Immune Defic Syndr. 2006 Oct 1;43(2):219–25. doi: 10.1097/01.qai.0000230526.79341.83. [DOI] [PubMed] [Google Scholar]
- 30.Stover J, Bollinger L, Hecht R, Williams C, Roca E. The impact of an AIDS vaccine in developing countries: a new model and initial results. Health Aff. 2007 Jul–Aug;26(4):1147–58. doi: 10.1377/hlthaff.26.4.1147. [DOI] [PubMed] [Google Scholar]
- 31.Bos JM, Postma MJ. The economics of HIV vaccines: projecting the impact of HIV vaccination of infants in sub-Saharan Africa. Pharmacoeconomics. 2001;19(9):937–46. doi: 10.2165/00019053-200119090-00005. [DOI] [PubMed] [Google Scholar]
- 32.Bogard E, Kuntz KM. The impact of a partially effective HIV vaccine on a population of intravenous drug users in Bangkok, Thailand: a dynamic model. J Acquir Immune Defic Syndr. 2002 Feb 1;29(2):132–41. doi: 10.1097/00042560-200202010-00004. [DOI] [PubMed] [Google Scholar]
- 33.Walensky RP, Paltiel AD, Goldie SJ, Gandhi RT, Weinstein MC, Seage GR, 3rd, et al. A therapeutic HIV vaccine: how good is good enough? Vaccine. 2004 Sep 28;22(29–30):4044–53. doi: 10.1016/j.vaccine.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 34.Barth-Jones DC, Cheng H, Kang LY, Kenya PR, Odera D, Mosqueira NR, et al. Cost effectiveness and delivery study for future HIV vaccines. AIDS. 2005 Sep 2;19(13):w1–6. doi: 10.1097/01.aids.0000181014.08127.a7. [DOI] [PubMed] [Google Scholar]
- 35.Ono S, Kurotaki T, Nakasone T, Honda M, Boon-Long J, Sawanpanyalert P, et al. Cost-effectiveness analysis of antiretroviral drug treatment and HIV-1 vaccination in Thailand. Jpn J Infect Dis. 2006 Jun;59(3):168–73. [PubMed] [Google Scholar]
- 36.Andersson KM, Owens DK, Vardas E, Gray GE, McIntyre JA, Paltiel AD. Predicting the Impact of a Partially Effective HIV Vaccine and Subsequent Risk Behavior Change on the Heterosexual HIV Epidemic in Low-and Middle-Income Countries: A South African Example. J Acquir Immune Defic Syndr. 2007;46(1):78–90. doi: 10.1097/QAI.0b013e31812506fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) Estimates of New HIV Infections in the United States. 2008 [cited September 18, 2008]; Available from: http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/incidence.htm.
- 39.Centers for Disease Control and Prevention (CDC) HIV/AIDS Surveillance Report, Volume 17. 2007 [cited September 18, 2008]; Available from: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2005report/default.htm.
- 40.Sanders GD, Bayoumi AM, Sundaram V, Bilir SP, Neukermans CP, Rydzak CE, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005 Feb 10;352(6):570–85. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 41.Panel on Clinical Practices for Treatment of HIV Infections. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Bethesda, Maryland: U.S. Department of Health and Human Services; 2006. [Google Scholar]
- 42.Yeni PG, Hammer SM, Hirsch MS, Saag MS, Schechter M, Carpenter CC, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004 Jul 14;292(2):251–65. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 43.Erb P, Battegay M, Zimmerli W, Rickenbach M, Egger M. Effect of antiretroviral therapy on viral load, CD4 cell count, and progression to acquired immunodeficiency syndrome in a community human immunodeficiency virus-infected cohort. Swiss HIV Cohort Study. Arch Intern Med. 2000 Apr 24;160(8):1134–40. doi: 10.1001/archinte.160.8.1134. [DOI] [PubMed] [Google Scholar]
- 44.The National Institute of Mental Health (NIMH) Multisite HIV Prevention Trial Group. The NIMH Multisite HIV Prevention Trial: reducing HIV sexual risk behavior. Science. 1998 Jun 19;280(5371):1889–94. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed] [Google Scholar]
- 45.Kamb ML, Fishbein M, Douglas JM, Jr, Rhodes F, Rogers J, Bolan G, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA. 1998 Oct 7;280(13):1161–7. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 46.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000 Mar 30;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 47.Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001 Mar 15;344(11):824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 48.Long EF, Brandeau ML, Galvin CM, Vinichenko T, Tole SP, Schwartz A, et al. Effectiveness and cost-effectiveness of strategies to expand antiretroviral therapy in St. Petersburg, Russia. AIDS. 2006 Nov 14;20(17):2207–15. doi: 10.1097/QAD.0b013e328010c7d0. [DOI] [PubMed] [Google Scholar]
- 49.Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral therapy on HIV-1 transmission and AIDS mortality in resource-limited settings. J Acquir Immune Defic Syndr. 2006 Apr 15;41(5):632–41. doi: 10.1097/01.qai.0000194234.31078.bf. [DOI] [PubMed] [Google Scholar]
- 50.McCormick AW, Walensky RP, Lipsitch M, Losina E, Hsu H, Weinstein MC, et al. The effect of antiretroviral therapy on secondary transmission of HIV among men who have sex with men. Clin Infect Dis. 2007 Apr 15;44(8):1115–22. doi: 10.1086/512816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007 Apr 17;146(8):591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 52.Joint United Nations Programme on HIV/AIDS (UNAIDS), World Health Organization (WHO). AIDS Epidemic Update, December 2007; 2007.
- 53.CensusScope. United States Age Distribution. 2000 [cited September 18, 2008]; Available from: http://www.censusscope.org/us/chart_age.html
- 54.Evans JL, Hahn JA, Page-Shafer K, Lum PJ, Stein ES, Davidson PJ, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO Study) J Urban Health. 2003 Mar;80(1):137–46. doi: 10.1093/jurban/jtg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman SR, Tempalski B, Cooper H, Perlis T, Keem M, Friedman R, et al. Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. J Urban Health. 2004 Sep;81(3):377–400. doi: 10.1093/jurban/jth125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rietmeijer CA, Wolitski RJ, Fishbein M, Corby NH, Cohn DL. Sex hustling, injection drug use, and non-gay identification by men who have sex with men. Associations with high-risk sexual behaviors and condom use. Sex Transm Dis. 1998 Aug;25(7):353–60. doi: 10.1097/00007435-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Greenwood GL, White EW, Page-Shafer K, Bein E, Osmond DH, Paul J, et al. Correlates of heavy substance use among young gay and bisexual men: The San Francisco Young Men's Health Study. Drug Alcohol Depend. 2001 Jan 1;61(2):105–12. doi: 10.1016/s0376-8716(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 58.Zaric GS, Barnett PG, Brandeau ML. HIV transmission and the cost-effectiveness of methadone maintenance. Am J Public Health. 2000 Jul;90(7):1100–11. doi: 10.2105/ajph.90.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005 Nov;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Desai K, Boily MC, Garnett GP, Masse BR, Moses S, Bailey RC. The role of sexually transmitted infections in male circumcision effectiveness against HIV--insights from clinical trial simulation. Emerg Themes Epidemiol. 2006;3:19. doi: 10.1186/1742-7622-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kral AH, Lorvick J, Ciccarone D, Wenger L, Gee L, Martinez A, et al. HIV prevalence and risk behaviors among men who have sex with men and inject drugs in San Francisco. J Urban Health. 2005 Mar;82(1 Suppl 1):i43–50. doi: 10.1093/jurban/jti023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harawa NT, Greenland S, Bingham TA, Johnson DF, Cochran SD, Cunningham WE, et al. Associations of race/ethnicity with HIV prevalence and HIV-related behaviors among young men who have sex with men in 7 urban centers in the United States. J Acquir Immune Defic Syndr. 2004 Apr 15;35(5):526–36. doi: 10.1097/00126334-200404150-00011. [DOI] [PubMed] [Google Scholar]
- 63.MacKellar DA, Valleroy LA, Behel S, Secura GM, Bingham T, Celentano DD, et al. Unintentional HIV exposures from young men who have sex with men who disclose being HIV-negative. AIDS. 2006 Aug 1;20(12):1637–44. doi: 10.1097/01.aids.0000238410.67700.d1. [DOI] [PubMed] [Google Scholar]
- 64.Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med. 2006 Sep 19;145(6):416–25. doi: 10.7326/0003-4819-145-6-200609190-00005. [DOI] [PubMed] [Google Scholar]
- 65.Tyndall MW, Patrick D, Spittal P, Li K, O'Shaughnessy MV, Schechter MT. Risky sexual behaviours among injection drugs users with high HIV prevalence: implications for STD control. Sex Transm Infect. 2002 Apr;78(Suppl 1):i170–5. doi: 10.1136/sti.78.suppl_1.i170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bacon O, Lum P, Hahn J, Evans J, Davidson P, Moss A, et al. Commercial sex work and risk of HIV infection among young drug-injecting men who have sex with men in San Francisco. Sex Transm Dis. 2006 Apr;33(4):228–34. doi: 10.1097/01.olq.0000204914.91923.ad. [DOI] [PubMed] [Google Scholar]
- 67.National Opinion Research Center. General Social Surveys (GSS), 1972–2006; 2006.
- 68.Fryar CD, Hirsch R, Porter KS, Kottiri B, Brody DJ, Louis T. Drug use and sexual behaviors reported by adults: United States, 1999–2002. Adv Data. 2007 Jun;28(384):1–14. [PubMed] [Google Scholar]
- 69.Johnson AM, Mercer CH, Erens B, Copas AJ, McManus S, Wellings K, et al. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet. 2001 Dec 1;358(9296):1835–42. doi: 10.1016/S0140-6736(01)06883-0. [DOI] [PubMed] [Google Scholar]
- 70.Brisson M, Boily MC, Masse BR, Adrien A, Leaune V. Highlights of the sexual activity of the heterosexual population in the province of Quebec. Sex Transm Infect. 1999 Oct;75(5):296–9. doi: 10.1136/sti.75.5.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spittal PM, Craib KJ, Wood E, Laliberte N, Li K, Tyndall MW, et al. Risk factors for elevated HIV incidence rates among female injection drug users in Vancouver. CMAJ. 2002 Apr 2;166(7):894–9. [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor A, Hutchinson S, Lingappa J, Wadd S, Ahmed S, Gruer L, et al. Severe illness and death among injecting drug users in Scotland: a case-control study. Epidemiol Infect. 2005 Apr;133(2):193–204. doi: 10.1017/s0950268804003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris ZK. Efficient allocation of resources to prevent HIV infection among injection drug users: the Prevention Point Philadelphia (PPP) needle exchange program. Health Econ. 2006 Feb;15(2):147–58. doi: 10.1002/hec.1021. [DOI] [PubMed] [Google Scholar]
- 74.Smith RJ, Blower SM. Could disease-modifying HIV vaccines cause population-level perversity? Lancet Infect Dis. 2004 Oct;4(10):636–9. doi: 10.1016/S1473-3099(04)01148-X. [DOI] [PubMed] [Google Scholar]
- 75.Arias E. United States Life Tables, 2001. Natl Vital Stat Rep. 2004 Feb 18;52(14):1–38. [PubMed] [Google Scholar]
- 76.Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993 Apr–Jun;13(2):89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 77.Holtgrave DR, Pinkerton SD. Updates of cost of illness and quality of life estimates for use in economic evaluations of HIV prevention programs. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 Sep 1;16(1):54–62. doi: 10.1097/00042560-199709010-00009. [DOI] [PubMed] [Google Scholar]
- 78.Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making. 2002 Nov–Dec;22(6):475–81. doi: 10.1177/0272989X02238300. [DOI] [PubMed] [Google Scholar]
- 79.Honiden S, Sundaram V, Nease RF, Holodniy M, Lazzeroni LC, Zolopa A, et al. The effect of diagnosis with HIV infection on health-related quality of Life. Qual Life Res. 2006 Feb;15(1):69–82. doi: 10.1007/s11136-005-8485-x. [DOI] [PubMed] [Google Scholar]
- 80.O'Donnell H. The United States Circumcision Century. 2001 [cited September 18, 2008]; Available from: http://www.boystoo.com/history/statistics.htm.
- 81.Bozzette SA, Joyce G, McCaffrey DF, Leibowitz AA, Morton SC, Berry SH, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med. 2001 Mar 15;344(11):817–23. doi: 10.1056/NEJM200103153441107. [DOI] [PubMed] [Google Scholar]
- 82.Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006 Nov;44(11):990–7. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 83.Gebo KA, Chaisson RE, Folkemer JG, Bartlett JG, Moore RD. Costs of HIV medical care in the era of highly active antiretroviral therapy. AIDS. 1999 May 28;13(8):963–9. doi: 10.1097/00002030-199905280-00013. [DOI] [PubMed] [Google Scholar]
- 84.Hutchinson AB, Farnham PG, Dean HD, Ekwueme DU, del Rio C, Kamimoto L, et al. The economic burden of HIV in the United States in the era of highly active antiretroviral therapy: evidence of continuing racial and ethnic differences. J Acquir Immune Defic Syndr. 2006 Dec 1;43(4):451–7. doi: 10.1097/01.qai.0000243090.32866.4e. [DOI] [PubMed] [Google Scholar]
- 85.World Health Organization (WHO) World Health Statistics. 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.