Abstract
Background and purpose:
We previously reported that NCX 2057, a compound comprising a nitric oxide (NO)-releasing moiety and the natural antioxidant, ferulic acid (FA), inhibits pro-inflammatory mediators through NO-mediated gene regulation. Here, we have assessed the activities of NCX 2057 in models of inflammatory and neuropathic pain, and characterized its effects on cyclooxygenase (COX)-1 and COX-2.
Experimental approach:
Anti-nociceptive and anti-inflammatory activities of NCX 2057 were measured in vitro and in vivo in models of inflammatory (carrageenan) and neuropathic (chronic constriction injury; CCI) pain. Effects of NCX 2057 were measured on COX-1 and COX-2 activities in RAW 264.7 macrophages.
Key results:
NCX 2057 dose-dependently inhibited single motor unit responses to noxious mechanical stimulation (ID50= 100 µmol·kg−1) and wind-up responses in rats with paw inflammation induced by carrageenan. Moreover, NCX 2057 inhibited allodynic responses following CCI of the sciatic nerve [ipsilateral Paw Withdrawal Threshold (g): vehicle: 41.4 ± 3.3; NCX 2057: 76.3 ± 4.8 FA: 37.9 ± 15.5 at 175 µmol·kg−1]. NCX 2057 reversed carrageenan-induced hyperalgesic responses in mice and inhibited prostaglandin E2 formation in paw exudates. Finally, NCX 2057 competitively inhibited COX-1 and COX-2 activities in whole RAW macophages (IC50= 14.7 ± 7.4 and 21.6 ± 7.5 µM, respectively). None of these properties were exhibited by equivalent treatments with FA or standard NO donor compounds.
Conclusions and implications:
These studies indicate that NCX 2057 is effective in chronic inflammatory and neuropathic pain models, probably because of its particular combination of anti-COX, antioxidant and NO-releasing properties.
Keywords: Ferulic acid, NCX 2057, nitric oxide, cyclooxygenase, inflammation, nociception
Introduction
Exaggerated sensitivity to pain is the dominant feature of inflammatory and neuropathic pain in clinical situations and experimental animal models. It is manifested as pain in response to innocuous stimuli (allodynia) or increased response to noxious stimuli (hyperalgesia), and persists long after the initial injury has resolved (Scholz and Woolf, 2007). Treatment of these types of pain relies on non-steroidal anti-inflammatory drugs, narcotic analgesics and anticonvulsants (such as gabapentin or carbamazepine) or antidepressant drugs, none of which are thought to exert disease-modifying properties.
An emerging theme in the pathophysiology of chronic and inflammatory pain is the role of pro-oxidant substances. Reactive oxygen species (ROS), including hydrogen peroxide, superoxide, hydroxyl radicals, nitric oxide (NO) and peroxynitrite, have been cited as molecules involved in the initiation and/or maintenance of chronic pain (Guedes et al., 2006; Figueroa-Romero et al., 2008). However, the role of NO in this situation is not clear, and seems to depend on its circulating concentration. For example, low concentrations of NO generated by the constitutive NO synthases, endothelial and neuronal, are considered beneficial, whereas high NO concentrations, derived from the activation of inducible NO synthase (iNOS), leads to oxidative stress, peroxynitrite formation, protein post-translational modifications and central sensitization of nociceptive circuits (Hancock and Riegger-Krugh, 2008). The mechanism of action underlying this sensitization seems to be linked to release of prostanoids (Ndengele et al., 2008).
Biosynthesis of prostanoids results from the activity of two well-recognized enzyme isoforms: cyclooxygenase (COX)-1, which is constitutively expressed in the majority of mammalian cells and is involved in homeostatic functions, and COX-2, which is induced during inflammation and following persistent activation of nociceptive circuits or cytokine exposure (Hoffmann, 2000; Svensson and Yaksh, 2002). There is also evidence for the existence of a constitutive expression of COX-2 (Maihofner et al., 2000; Seybold et al., 2003) and a third isoform, COX-3, whose function is still not well understood, has also been described (Warner and Mitchell, 2002; Simmons et al., 2004).
Various non-selective as well as selective COX-2 inhibitors have been shown to counteract hyperalgesic responses in a variety of experimental conditions (Patrignani et al., 2005) and exert prominent anti-inflammatory activity (Ibuki et al., 2003; Burian and Geisslinger, 2005). On the other hand, a variety of antioxidant compounds, including ferulic acid (FA) and its closely related derivative, curcumin, have been shown to inhibit COX-2 and iNOS protein expression, as well as the production of pro-inflammatory cytokines (Hosoda et al., 2002; Dong et al., 2003; Sharma et al., 2006; Aggarwal and Sung, 2009). Furthermore, recent pre-clinical reports suggest that various new and old antioxidants are effective anti-nociceptive agents (Tankova et al., 2005; Sharma et al., 2006; Valsecchi et al., 2008). These latter effects are shared by slow NO-releasing compounds. Indeed, the introduction of an NO-releasing moiety into the chemical structure of various drugs has consistently been shown to provide anti-inflammatory properties and to inhibit pro-inflammatory enzymes and mediators (Wu et al., 2004; Ongini et al., 2004; Ronchetti et al., 2006). These and other observations suggest that drugs targeting COX inhibition, free radical formation and pro-inflammatory protein expression (e.g. iNOS) might provide a better control over inflammatory and neuropathic pain conditions than the therapeutic agents currently available.
We and others have previously reported that the NO-releasing derivative of the natural antioxidant FA, NCX 2057, retains antioxidant activity similar to that of its parent compound, FA (Wenk et al., 2004), inhibits iNOS expression through the nuclear factor-κB signalling pathway (Ronchetti et al., 2006) and inhibits microglia activation in a rat model of chronic neuroinflammation (Wenk et al., 2004).
Based on these observations, we studied the anti-inflammatory, anti-hyperalgesic and anti-allodynic activities of NCX 2057 in well-established rodent models of pain. We also assessed whether these possible actions were located in peripheral tissues or within the spinal cord by administering the compound by different routes. In addition, we characterized its inhibitory activity on COX-1 and COX-2 enzymes.
Our data suggest that NCX 2057 is an effective anti-nociceptive compound in models of inflammatory or neuropathic pain. The effect is mainly located in peripheral tissues, though some activity in the spinal cord cannot be discounted. These results support the effectiveness of compounds like NCX 2057 in nociception and might open new perspectives in the treatment of pain.
Methods
Animals
All animal care and experiments in this study were undertaken in accordance with Italian and European legislation regarding the use of animals for experimental protocols and all efforts were made to minimize animal suffering and to reduce the number of animals used. Carrageenan-induced hyperalgesia was studied in male CD-1 mice weighing 25–30 g. Behavioural studies and electrophysiological experiments were carried out in male Wistar rats weighing 235–350 g. Animals were only used for a single experiment and were killed by an overdose of sodium pentobarbital (Dolethal, Vetoquinol S.A., Madrid, Spain) at the end of each experimental session.
Cell culture
RAW 264.7 mouse macrophage cells were maintained at 37°C in presence of 95% air and 5% CO2 in Dulbecco's modified Eagle's medium (Invitrogen, PA, USA) containing 10% (v/v) fetal bovine serum, 25 000 units·mL−1 penicillin, 25 mg·mL−1 streptomycin and 4 mM L-glutamine (Gibco, Grand Island, NY, USA). Before each individual experiment, cells were rinsed and incubated in serum-free medium for 16 h (resting conditions for COX-1 activity) or in presence of 1 µg·mL−1 of the bacterial endotoxin, lipopolysaccharide (LPS) and 10 ng·mL−1 of interferon-γ (IFNγ) (to induce COX-2 activity). LPS and IFNγ were from Sigma-Aldrich (St. Louis, MO, USA) and Roche Molecular Biochemicals (Mannheim, Germany) respectively.
Electrophysiological and behavioural studies
Electrophysiological recording in carrageenan-induced paw inflammation in rats
The method followed has been described previously (Solano and Herrero, 1997; Romero-Sandoval et al., 2004). Briefly, the experiments were carried out in male Wistar rats, injected in the right hind paw (intra-plantar) with 100 µL of 1% λ-carrageenan solution (Sigma-Aldrich). Preparative surgery (e.g. cannulation of the trachea, of the carotid artery and of the two superficial branches of the jugular veins) was performed under halothane anaesthesia. The preparation was left to rest for at least 1 h prior to the experimental session, which was carried out under general anaesthesia with α-chloralose (50 mg·kg−1 initial dose and 20 mg·kg−1 h−1 for maintenance; Sigma-Aldrich) and terminated with an overdose of sodium pentobarbital. The right hind limb was fixed in inframaximal extension in a Perspex block using plaster of Paris. The activity of single motor units (SMUs) was recorded by means of tungsten bipolar electrodes inserted percutaneously into muscles of the hind limb. Throughout the experiments, blood pressure and core temperature were monitored and maintained within normal physiological limits. SMU activity was recorded from hind limb muscles using a Teflon-coated tungsten electrode (Solano and Herrero, 1997) and a standard electrophysiological setup. The units were activated in 3 min cycles (10 s noxious mechanical stimulation and one train of 16 percutaneous electrical stimuli). Noxious mechanical stimulation was applied over an area of 14 mm2 using a computer-controlled pincher device (Estimec, Cibertec, Madrid, Spain), and a force of 200 mN over the threshold intensity, threshold being the minimal pressure required to evoke a constant firing rate (Herrero and Headley, 1991; Solano and Herrero, 1997). The electrical stimulation was applied using two 0.2 mm needles inserted in the most sensitive area of the cutaneous receptive field, with 16 pulses of 2 ms width, 1 Hz and an intensity of twice the threshold current for C-fibre responses (Herrero and Cervero, 1996). The drugs were tested only when the responses observed with either stimulus were stable.
FA and NCX 2057 (NicOx S.A.) were prepared fresh everyday, immediately before use. The drugs were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) and polyethylene glycol (1:1; Panreac, Barcelona, Spain) in a concentration of 50 mM, diluted in saline and administered intravenously in cumulative log2 regime in a total and constant volume of 0.3 mL. The initial dose used was 25 µmol·kg−1 and the highest dose used was 200 µmol·kg−1. Preliminary experiments showed that peak effect of NCX 2057 was observed within the first 5 min after administration. Accordingly, the doses studied were given every 6 min (2 cycles of stimulation).
The collection of data and stimulation protocols were performed by a computer using commercial software (Spike 2; CED, Cambridge, UK). The number of spikes counted in the last two cycles of stimulation between each dose were averaged and the mean compared to the control response, control being the mean of the three responses previous to the administration of the first dose (see for further details Herrero and Headley, 1991; Solano and Herrero, 1997). Spikes from mechanical and electrical stimulation were counted and analysed separately. The data from the electrical stimulation were analysed by counting the number of spikes evoked between 150 and 650 ms after each stimulus (Herrero and Cervero, 1996).
Carrageenan-induced paw inflammation in mice
λ-Carrageenan (10 mg·mL−1 in saline; Sigma-Aldrich) was dissolved by sonication and injected subcutaneously in a volume of 20 µL into the right plantar hind paw using a 50 µL microsyringe with a 27 gauge needle. Drug or vehicle was dissolved directly in the λ-carrageenan solution just before the injection. Mice were then placed under a transparent plastic box (5 × 5 × 4 cm) on a metal mesh floor. Mechanical sensitivity was measured after 4 h by determining the median 50% paw withdrawal threshold (PWT) with von Frey monofilaments (bending force 0.02, 0.06, 0.16, 0.4, 1.2, 2.04 and 3.6 g; applied using a single, steady >1 s application) using the up/down method (Chaplan et al., 1994).
Prostaglandin E2 (PGE2) production in carrageenan-injected mouse paws
Mice from different groups were killed 4 h after λ-carrageenan administration. Paws were dissected and centrifuged at 4000 ×g for 30 min at 4°C to obtain the inflammatory exudates. Supernatant (5 µL) was collected and diluted with 10 volumes of ice-cold phosphate buffer saline (PBS), the protein content was quantified by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL, USA) and finally, the levels of PGE2 were determined by PGE2 metabolite EIA-kit purchased from Cayman Chemical (Ann Arbor, MI, USA) following deproteinization of the samples with a solution of ZnSO4 (30% v/v).
Chronic constriction injury (CCI)-induced mechanical allodynia in rats
The method described by Bennet and Xie (1988) was generally followed. Rats were anaesthetized with chloral hydrate (380 mg·kg−1ip, Sigma-Aldrich). The right common sciatic nerve was exposed at the level of the femoris by blunt dissection. Proximal to the sciatic nerve trifurcation, about 12 mm of nerve was freed of adhering tissue and four ligatures (3/0 silk suture) were tied loosely around it with about 1 mm spacing. Ligatures were tied such that the diameter of the nerve was only barely constricted. The desired degree of constriction retarded, but did not arrest, circulation through the superficial epineural vasculature. The incision was closed in layers. The experiments were then carried out 1 week after this surgical preparation of the animals.
Mechanical sensitivity was determined with an analgesimeter (Ugo Basile, Varese, Italy), using the modified Randall–Selitto method. Briefly, a conical stylus with a hemispherical tip was placed upon the middle of hind paw dorsum. The animal was gently restrained and calibrated pressure of gradually increasing intensity was applied until the rat withdrew the hind paw. Threshold pressure (g) of both hind paws was determined every 15 min, with two measurements before the treatments (pre-test), and from 15 to 60 min after treatment. An arbitrary cut-off value of 240 g was adopted. All the compounds were given by s.c. injection.
Biochemical studies
Western blot analysis
RAW 264.7 cells (5–10 105) were scraped off the plates in ice-cold PBS with a rubber cell lifter and centrifuged at 3800×g for 5 min. The cell pellets were dissolved in 250 µL of 0.5% (v/v) Triton-X100, 0.5% (v/v) sodium deoxycholate (SDS, Sigma-Aldrich) in PBS (Gibco) containing a mixture of protease inhibitors (Roche, Basel, Switzerland) and spun at 21 000×g for 10 min. Protein content in cell lysates was assayed by BCA assay (Pierce). An equal amount of proteins from each sample were loaded onto 4–12% SDS polyacrylamide gel electrophoresis (SDS-PAGE gel), transferred to polyvinylidene fluoride membrane (Millipore, MA, USA) and immuno-stained using an anti-COX-2 (Upstate Biotechnology, NY, USA) polyclonal antibody or anti-β-actin (Cell Signaling Technology Inc., MA, USA), followed by a horseradish peroxidase-conjugated anti-rabbit secondary antibody. Bands were visualized using chemiluminescence reagents (Perkin Elmer, Wellesley, MA, USA). Band intensity was determined using a ChemiDoc system (Bio Rad, Hercules, CA, USA) and the Quantity One software (version 4.3.1, Bio Rad). For quantitative analysis, the specific bands were normalized on β-actin signals and data were expressed as a percentage of LPS/IFNγ-stimulated levels ± standard error of mean (SEM).
COX-1 COX-2 activities in macrophages
COX-1 and COX-2 activities were studied using resting and LPS/IFNγ-stimulated RAW 264.7 cells respectively. Before the experiment, cells were rinsed and incubated for 8 h in serum-free medium with or without 100 µM of aspirin (respectively for COX-2 and COX-1 activity determination). Subsequently, COX-2 expression was induced by 16 h (overnight) incubation with 1 µg·mL−1 LPS and 10 ng·mL−1 IFNγ. The next day, cells were collected, resuspended in serum-free media and incubated with drugs or vehicle for 30 min at 37°C. At the end of the incubation time, 1 µM of the enzyme substrate, arachidonic acid (AA), was added and the cells were further incubated at 37°C for 15 min. The reaction was ended with 3% of trichloroacetic acid and the samples filtered. The extent of compound-induced inhibition was determined by assessing the amount of PGE2 release into the incubation buffer using a PGE2 EIA-kit purchased from Cayman Chemical. Individual readings (PGE2, ng·mL−1) were corrected for the number of cells in each experiment (about 105 cells per sample) and, subsequently, converted to respective percent of control values (PGE2, % of control). The latter values [PGE2, % of control = (y) in the equation below] were computed and fitted as a function of concentration [µM = (x) in the equation below] into a 4 parameters logistic curve using the following equation: y = y0+[a (1 + (x/x50)b)−1], where y0= minimum (y), a = maximum (y) and b = slope. The IC50 (x50, in the equation above) values ± SEM were then estimated from the resulting plots using Sigma-Plot regression analysis.
The kinetic parameters of the reaction were determined by incubating resting or stimulated cells with various concentrations of AA (ranging from 0.15 to 10 µM) in presence of fixed concentration of NCX 2057, FA or vehicle and the velocity of the reactions was quantified. Km and Vmax values were determined by the analysis of the resulting Lineweaver–Burke plot. Ki values of NCX 2057 for COX-1 and COX-2 were calculated from the following equation: Ki =[test drug]× (α-1)−1 where α represents the ratio between the slope obtained from the Lineweaver–Burke plots in presence and absence of the test drug.
COX-1 and COX-2 activities in isolated ovine recombinant enzymes
The assay was developed from the commercially available ovine COX-inhibitor screening assay (Cayman Chemical). AA was added at the final concentration of 1 µM.
Data analysis
Results are presented as mean ± SEM. Statistical analysis for in vivo and in vitro assays used one-way anova followed by Dunnett's post hoc test unless otherwise specified. P values of less than 0.05 were considered significant.
Comparison of ID50s was made with the two-tail unpaired t-test.
Results
Electrophysiological and behavioural studies
Carrageenan-induced mechanical hyperalgesia in rats
As described in previous experiments (Romero-Sandoval et al., 2004), the injection of 100 µL λ–carrageenan into the right hind paw of rats elicited obvious tissue inflammation associated with mechanical hyperalgesia that peaked 4 h after induction and lasted for at least 48 h. Experiments were performed at the plateau stage, e.g. 16 h after the injection. The intravenous administration of cumulative doses of NCX 2057 (from 10 to 200 µmol·kg−1, n= 6) induced a dose-dependent reduction of the nociceptive responses, measured as the electrical activity of SMUs from the affected paw. The maximal effect of NCX 2057 reduced SMU activity to about 30% of the control response, with a calculated ID50 of 100 µmol·kg−1 (Figure 1). Under the same experimental conditions, the administration of cumulative doses of FA (n= 5) did not significantly modify SMU responses to noxious mechanical stimulation (Figure 1).
Figure 1.
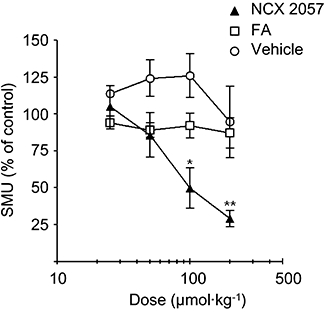
Effects of NCX 2057 on responses to noxious mechanical stimulation in carrageenan-induced paw inflammation in rats. NCX 2057 inhibited dose-dependently single motor unit (SMU) responses evoked by noxious mechanical stimulation with an ID50 of 100 µmol·kg−1, whereas the administration of ferulic acid (FA) or vehicle was ineffective. Compounds or vehicle were administered i.v. in a cumulative manner from 25 to 200 µmol·kg−1. Data are presented as mean ± standard error of mean. *P < 0.05 versus control; **P < 0.01 versus control.
In three additional experiments, pre-treatment with a fully effective dose (1 mg·kg−1, i.v.) of naloxone did not alter the effects of NCX 2057 (data not shown), suggesting that NCX 2057 acts through an opioid-independent mechanism. Control experiments (n= 3) studying the equivalent amounts of vehicle used in the studies showed no significant reduction of nociceptive responses (Figures 1 and 2).
Figure 2.
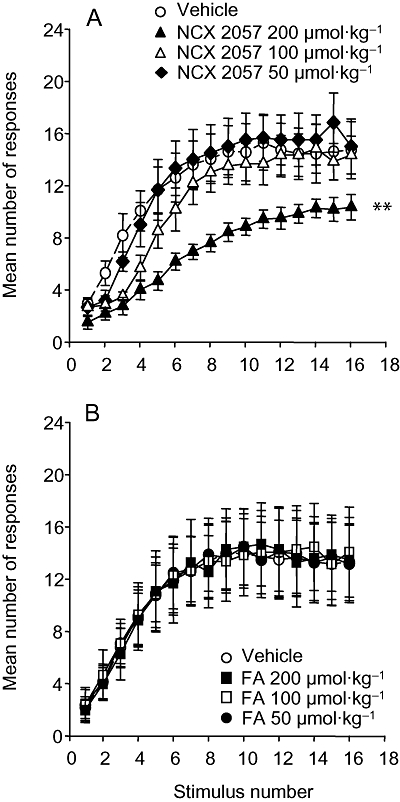
Effects of NCX 2057 and ferulic acid (FA) on wind-up responses in carrageenan-induced paw inflammation in rats. The figure shows the effects of vehicle (upper and lower panels), NCX 2057 (upper panel) at doses of 200 µmol·kg−1, 100 µmol·kg−1 and 50 µmol·kg−1 or FA (lower panel; also 200–50 µmol·kg−1). All the compounds were administered i.v. Data represent mean ± standard error of mean. **P < 0.01 versus vehicle curve.
Carrageenan-induced electrical wind-up in rats
In animals with one paw inflamed with carrageenan, a progressive increment of the number of spikes (wind-up response) was observed following repeated low-intensity electrical stimulation. The intravenous administration of NCX 2057 induced a dose-dependent reduction of wind-up, though a significant depression was only observed with the highest dose studied (200 µmol·kg−1) (Figure 2A). Conversely, the administration of cumulative doses of FA (up to 200 µmol·kg−1) did not significantly modify the wind-up response (Figure 2B). As observed in the assays for SMU activity from noxious mechanical stimulation, the effects of NCX 2057 on wind-up were not altered by the i.v. administration 1mg kg−1 of the opioid receptor antagonist, naloxone (data not shown).
CCI-induced mechanical allodynia in rats
Seven days after sciatic nerve ligation, the mechanical PWT of the CCI-lesioned ipsilateral paw (ipsi) was stable and significantly lower (pre-test, Figure 3A) than that of the respective contralateral, un-lesioned paw (contra; Figure 3B). Subcutaneous administration of NCX 2057 significantly increased ipsi PWT in a dose-dependent manner with a profile similar to that observed following the administration of gabapentin at equimolar doses which was used as internal positive control in this set of experiments (Figure 3A and C). NCX 2057 also enhanced PWT contralateral to the lesion side when administered at 175 µmol·kg−1 (Figure 3B and D). The effects were already evident at 15 min, remained stable during the following 30 min and decreased thereafter (Figure 3A and B). Conversely, the administration of equimolar doses of FA was virtually ineffective (Figure 3A–D). The effects observed may not be ascribed to a general depressant or stimulating effect on locomotor activity, as NCX 2057 given to rats at doses up to 580 µmol·kg−1 did not elicit any appreciable impairment or enhancement of their rotarod performance (data not shown).
Figure 3.
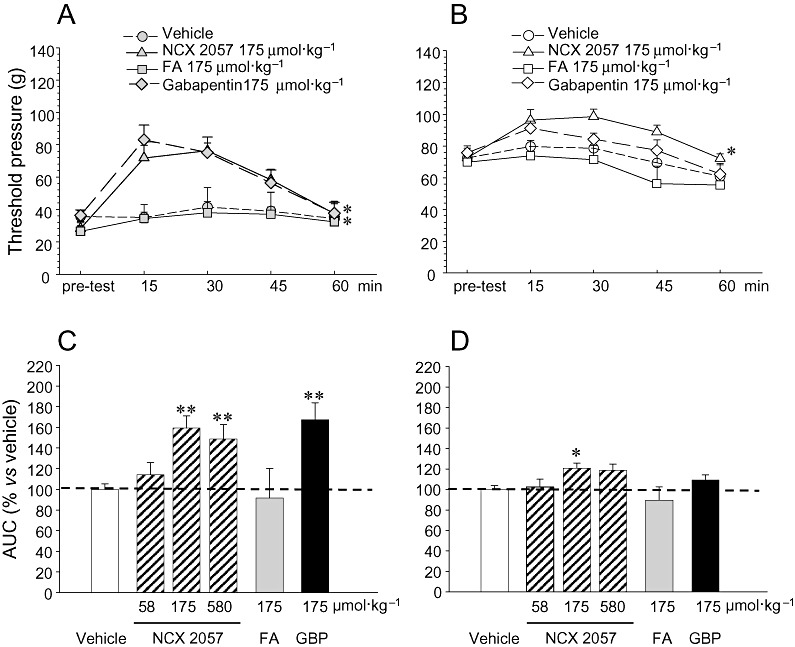
Effects of NCX 2057 and ferulic acid (FA) on mechanical allodynia in rats with chronic constriction injury (CCI). Effects of vehicle, NCX 2057 (175 µmol·kg−1), FA (175 µmol·kg−1) or gabapentin (175 µmol·kg−1) on nociceptive threshold [as paw withdrawal threshold (PWT)] in ipsilateral-CCI paws are shown in A and from contralateral non-lesioned paws in B. In C and D, these effects are expressed as area under curve (AUC) from the time courses shown in A and B. For NCX 2057, FA, gabapentin (GBP) and vehicle in CCI paws, data are shown in C. Corresponding data for the non-lesioned, contralateral paw are shown in D. Compounds were given by s.c. injection and mechanical threshold was measured with an analgesimeter (modified Randall–Selitto method) before treatment (pre-test) and from 15 to 60 min after treatment. In panel B, PWT responses over time obtained following individual treatments were integrated (AUC) and are presented as percent of response to vehicle. Data represents mean ± standard error of mean of 6–8 animals for each experimental group. **P < 0.01 versus respective vehicle treatment.
Carrageenan-induced mechanical hyperalgesia in mice
The effects of NCX 2057 on mechanical hyperalgesia were studied in mice with one paw inflamed with λ-carrageenan. As seen in rats, the injection of carrageenan into the plantar hind paw of mice resulted in rapid (within 30 min) inflammatory response associated with profound tissue swelling. Mechanical hyperalgesia developed slowly during the following hours reaching a maximum by 4 h after treatment. This time was then chosen to study the effects of NCX 2057. Responses to von Frey filaments, as shown by the 50% PWT, decreased dramatically in carrageenan-injected paw, compared to that observed following sham injection (Figure 4A). However, as also shown in Figure 4A, the carrageenan-induced hyperalgesic responses were reversed, in a dose-dependent manner by local intraplantar injection of NCX 2057, but not that of FA (Figure 4A). When given systemically, by s.c. injection, NCX 2057, but not FA, at doses as high as 580 µmol·kg−1 inhibited these responses to much lower extent (50% threshold: 0.70 ± 0.10 g, P < 0.05 vs. vehicle).
Figure 4.
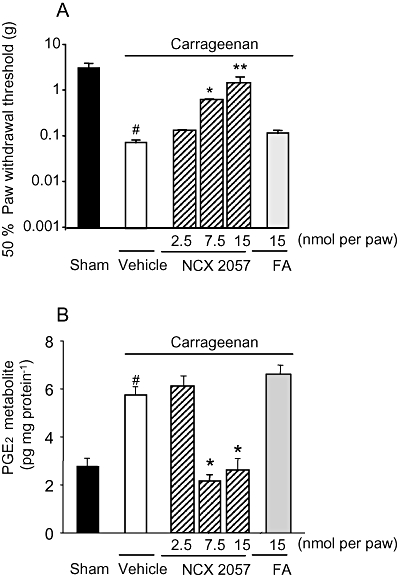
Effects of NCX 2057 and ferulic acid (FA) on mechanical hyperalgesia and prostaglandin E2 (PGE2) formation in carrageenan-induced inflammation in mice. (A) Responses to mechanical stimulation with von Frey filaments and (B) contents of PGE2 metabolites in paw exudates were determined in sham or carrageenan injected animals treated locally with vehicle NCX 2057 or FA at the indicated doses. Mechanical sensitivity and PGE2 formation, estimated by determination of the two major metabolites, 13, 14-dihydro-15-keto PGE2 and 13, 14-dihydro-15-keto PGA2 were determined 4 h after carrageenan injection. Data represent mean ± standard error of mean of 8–10 animals for each experimental group. *P < 0.05, **P < 0.01 versus vehicle treatment, #P < 0.05 versus sham treatment. In this set of experiments, naproxen, administered orally as a positive control, had an ID50 of 2.3 mg·kg−1.
Effects of NCX 2057 on PGE2 production in carrageenan-injected mouse paw
We next studied whether the anti-hyperalgesic effects of NCX 2057 were accompanied by direct activity on PGE2 production in carrageenan-treated mouse paws. The intraplantar injection of λ–carrageenan increased the PGE2 content of paw exudates (Figure 4B). This increase was inhibited in a dose-dependent manner by the concomitant injection of NCX 2057 (2.5, 7.5 and 15 nmol per paw) (Figure 4B). However, FA, up to 15 nmol per paw, did not significantly modify carrageenan-induced PGE2 synthesis (Figure 4B).
Biochemical studies
Effects of NCX 2057 on COX-1 and COX-2 expression and activity
Exposure to FA or NCX 2057 at dose up to 50 µM failed to significantly alter COX-2 protein expression in LPS/IFNγ-stimulated RAW 264.7 macrophages (Figure 5A and B) while NCX 2057 reduced PGE2 formation in culture media. More specifically, NCX 2057 inhibited in a concentration-dependent manner the COX-1 and COX-2 activities of RAW macrophages with very similar potencies (Figure 6A and B; Table 1). By contrast, FA was inactive up to 100 µM (Figure 6A and B; Table 1). Similar experiments were also conducted in the presence of porcine liver esterases (1.7 units per sample) known to readily metabolize NCX 2057 (Govoni et al., 2006). Under these conditions, NCX 2057 lost its inhibitory activity on either COX isoform (Table 1). Moreover, various NO donors (e.g. S-nitrosoglutathione, S-nitroso-N-acetyl-penicillamine and diethylenetriamine NONOate) at concentrations ranging from 1 to 100 µM failed to significantly alter COX-1 and COX-2 function (Table 1) suggesting that the effects of NCX 2057 may not be ascribed to NO release.
Figure 5.
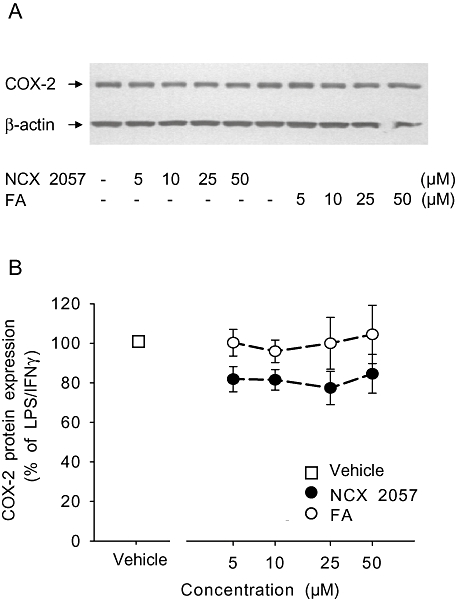
Effects of NCX 2057 and ferulic acid (FA) on lipopolysaccharide (LPS)/ interferon-γ (IFNγ)-stimulated inducible nitric oxide synthase protein expression. NCX 2057, FA or vehicle were added to the RAW macrophages 30 min before the overnight activation with LPS/IFNγ. (A) Representative Western blot of cyclooxygenase type-2 (COX-2) and β-actin proteins. (B) Quantitative analysis of the protein expression levels normalized to the respective β-actin content. Data represent mean ± standard error of mean.
Figure 6.
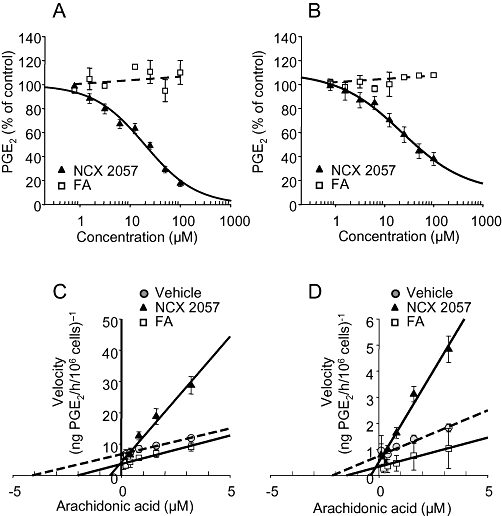
Effects of NCX 2057 and ferulic acid (FA) on cyclooxygenase type-1 (COX-1) and cyclooxygenase type-2 (COX-2) activities in RAW macrophages. Panels A and B show the inhibitory profile of NCX 2057 and FA on COX-1 and COX-2 respectively. Values represent the mean ± standard error of mean of three independent experiments each conducted in triplicate. Experiments were performed in presence of 1 µM of the enzyme substrate, arachidonic acid and the extent of drug-induced inhibition was estimated from the amount of prostaglandin E2 (PGE2) production. Panels C and D are representative Lineweaver–Burke plots for COX-1 and COX-2 enzymes in the absence, in the presence of NCX 2057 at 30 µM or FA at 30 µM.
Table 1.
Inhibitory potency of NCX 2057, FA, naproxen and S-nitrosoglutathione on COX-1 and COX-2
| Treatment |
RAW 264.7 mouse macrophages |
Isolated ovine enzymes |
||
|---|---|---|---|---|
| COX-1 IC50 (µM±SEM) | COX-2 IC50 (µM±SEM) | COX-1 IC50 (µM±SEM) | COX-2 IC50 (µM±SEM) | |
| NCX 2057 | 14.7 ± 7.4 | 21.6 ± 7.5 | 13.06 ± 2.8 | 33.6 ± 3.5 |
| NCX 2057 + esterasesb | >100a | >100a | >100a | >100a |
| FA | >100a | >100a | >100a | >100a |
| FA + esterasesb | >100a | >100a | >100a | >100a |
| Naproxen | 13.5 ± 4.1 | 7.4 ± 2.0 | 1.66 ± 0.8 | 3.3 ± 0.5 |
| S-nitrosoglutathione | >100a | >100a | n.d. | n.d. |
The compound elicited only marginal inhibition (below 20%) at the highest concentration tested (100 µM). Naproxen was used as positive control; S-nitrosoglutathione was used as standard nitric oxide donor. Similar results were obtained with S-nitroso-N-acetyl-penicillamine and Diethylenetriamine NONOate.
Porcine liver esterases (1.67 units) were used.
COX-1, cyclooxygenase type-1; COX-2, cyclooxygenase type-2; FA, ferulic acid; n.d., not determined; SEM, standard error of mean.
To better understand the mechanism of action of NCX 2057-mediated COX inhibition, we studied the kinetics of COX-1 and COX-2 enzymic activities in presence and absence of various concentrations of NCX 2057 or FA. As shown in Figure 6C and D and Table 2, the Vmax and Km values for COX-1 or COX-2 were not significantly altered by FA (30 µM) However, incubation with the same concentration of NCX 2057 very clearly increased the Km values for both COX-1 and COX-2 (Figure 6C and D; Table 2; P < 0.05 vs. vehicle). Similar effects were also observed at lower concentrations. As shown in the Lineweaver–Burke plots, NCX 2057 did not significantly affect the Vmax of either COX-1 or COX-2 (Figure 6C and D; Table 2). This profile is consistent with a competitive interaction of NCX 2057 with COX enzymes. The equilibrium constants for the dissociation of NCX 2057 from COX-1 and COX-2 were, respectively, KiCOX-1= 5.1 ± 1.1 µM and KiCOX-2= 11.0 ± 3.2 µM.
Table 2.
Effects of NCX 2057 and FA on COX-1 and COX-2 enzyme kinetics
| Treatment | COX-1 | COX-2 | ||
|---|---|---|---|---|
| Km (µM) | Vmax (ng PGE2 h−1 10-6 cells) | Km (µM) | Vmax (ng PGE2 h−1 10-6 cells) | |
| Vehicle | 0.23 ± 0.11 | 0.28 ± 0.12 | 0.39 ± 0.10 | 1.47 ± 0.29 |
| NCX 2057 (30 µM) | 2.74 ± 1.05* | 0.34 ± 0.10 | 1.68 ± 0.55* | 1.45 ± 0.20 |
| FA (30 µM) | 0.34 ± 0.02 | 0.26 ± 0.03 | 0.54 ± 0.03 | 1.82 ± 0.07 |
The equilibrium constant for the dissociation of NCX 2057 (Ki) from COX-1 and COX-2 estimated from enzyme kinetics following exposure to various concentrations of NCX 2057 were respectively: Ki COX-1= 5.1 ± 1.1 µM and Ki COX-2= 11.0 ± 3.2 µM.
P < 0.05 versus vehicle treated group.
FA, ferulic acid; COX-1, cyclooxygenase type-1; COX-2, cyclooxygenase type-2; PGE2, prostaglandin E2; Data represent mean ± standard error of mean.
Finally, to rule out potential indirect effects of NCX 2057, we also performed similar analysis using isolated recombinant ovine COX-1 and COX-2 enzymes yielding similar results (Table 1).
Discussion and conclusions
The main observation made in the present study is the significant reduction of nociceptive responses and inflammation exhibited by NCX 2057 in rodents. This action was evident in mice or rats, and after intravenous, subcutaneous or intraplantar administration. This suggests not only a fast anti-nociceptive activity but also a mechanism of action located in the inflamed tissue, for example a peripheral mechanism of action.
Plantar injection of carrageenan rapidly evokes local erythema and oedema later accompanied by a decreased threshold to mechanical stimuli (mechanical hyperalgesia). This initial phase is thought to depend primarily on the release of peripheral inflammatory mediators, including bradykinins, inflammatory cytokines and prostaglandins (Poole et al., 1999). Consistent with this, local induction of COX-2 and PGE2 formation within 2–4 h after carrageenan pleurisy has been reported (Guay et al., 2004). Likewise, gene disruption of PGE2 receptors in mice or the direct injection of monoclonal PGE2 antibody has been shown to inhibit the early phase of carrageenan-induced inflammatory response and pain (Portanova et al., 1996; Reinold et al., 2005). In our study, we found a robust local increase of PGE2 production following carrageenan-induced paw inflammation that was inhibited by NCX 2057, supporting the notion that this compound acts, at least in part, by direct inhibition of local COX enzymes.
Systemic (i.p. or s.c.) exposure of NCX 2057 at doses up to 580 µmol·kg−1, albeit significantly, only resulted in moderate effects in mice. The lower efficacy of NCX 2057, when administered systemically in this model, may well depend on its rapid metabolism that would decrease the amounts of the intact compound reaching the inflamed paw, though It might also mean a lack of a central action.
We also observed that intravenous NCX 2057 counteracted spinal cord nociceptive responses to mechanical stimulation and wind-up in rats with paw inflammation. However, the effect on the activity evoked by noxious mechanical stimulation was more intense (and as effective as that observed after local injection) than that observed in wind-up responses. As wind-up is a centrally mediated phenomenon, the low intensity of effect observed after the administration of NCX 2057 supports an action located mainly at peripheral sites (see Herrero et al., 2000 for further discussion on this subject). In this case, the compound was injected intravenously and, in contrast to subcutaneous administration, it could reach the target tissue more easily and rapidly. Nevertheless, since a small but significant effect was observed in wind-up responses, a partial action within the spinal cord nociceptive processing cannot be discounted. The mechanisms of action involved in this centrally mediated effect are the aim of future experiments. However, a depression of the mechanisms underlying the wind-up phenomenon including modulation of D-methyl-Aspartic acid and neurokinin-1 receptors (Herrero et al. 2000) is likely, though other possibilities, have to be taken in consideration.
Non-steroidal anti-inflammatory drugs (NSAIDs) have proven to be the drugs of choice for the treatment of chronic inflammatory pain (Kean and Buchanan, 2005). The therapeutic activity of this class of compounds has generally been attributed to their established ability to inhibit COXs, key enzymes in prostanoid biosynthesis (Vane, 1971; Vane et al., 1998; Simmons et al., 2004).
In this study, NCX 2057 inhibited COX activities in whole cells as well as in isolated recombinant enzymes without changes in the expression of the COX proteins. Kinetic analysis of COX enzymes in presence and absence of NCX 2057 demonstrated that this compound was a reversible, non-selective and competitive inhibitor on both COX-1 and COX-2 enzyme isoforms. These effects could not be ascribed to parent FA and/or to the NO-releasing moiety alone as we observed that equivalent concentrations of FA and NO-donors were inactive in this assay. In addition, NCX 2057 was devoid of any activity when pre-exposed to liver esterases, which rapidly cleave the ester bond bridging FA to the NO-releasing moiety (Govoni et al., 2006).
All these results indicated that an inhibitory action of NCX 2057 on COX activity in the spinal cord is possible. Several studies, for example, indicate that COX-2-mediated increase of PGE2 in the spinal cord and central nervous system (CNS) contributes to the severity of pain responses in the late phase of carrageenan-induced hyperalgesia. In fact, COX-2 mRNA and protein expression, as well as PGE2 formation, have been observed in spinal cord neurons 24 h after the injection of carrageenan (Guay et al., 2004). This suggests that the centrally mediated effect of NCX 2057 may be related to an inhibition of this signalling pathway in the spinal cord. This is also in line with the depression of nociceptive spinal cord neuronal activity observed in our experiments (see Herrero et al., 2000 for further discussion in this subject). In fact, we and others have previously shown that many COX inhibitors depress spinal cord nociceptive activity and wind-up using similar experimental models (Herrero et al., 2000). For instance, indomethacin and SC58125, respectively a non-selective and a selective COX-2 inhibitor, have been shown to inhibit wind-up activity in a dose-dependent manner (Willingale et al., 1997). Thus far, the tissue distribution and metabolic fate of NCX 2057 following systemic in vivo exposure have not been addressed in this study. In spite of that, Wenk et al. (2004) reported that NCX 2057 inhibits microglia activation in a rat model of neuroinflammation, indicating the ability of this compound to modulate central functions.
In previous studies, NCX 2057 has been shown to release significant amount of NO over time and activate the NO/cGMP signalling pathway (Govoni et al., 2006; Ronchetti et al., 2006). To what extent this contributed to the anti-hyperalgesic/anti-allodynic effects of NCX 2057 remains difficult to address since the specific role of NO/cGMP signalling in peripheral tissue and the CNS is highly controversial. Whereas some studies suggest that central activation of the NO/cGMP pathway leads to the modulation of Gamma-aminobutyric acid and glycine inhibition of spinothalamic tract neurons and central sensitization (Lin et al., 1999), others document an NO-mediated anti-nociceptive effect viaβ-endorphin-induced release of methionine-enkephalin in the spinal cord (Hara et al., 1995). On the other hand, peripheral activation of NO/cGMP signalling mainly translate into an anti-hyperalgesic effect (Durate et al., 1990; Jain et al., 2001) and thus, it is more likely to have contributed to the overall effects of NCX 2057.
An even more intriguing finding of this study is the observation that the systemic exposure to NCX 2057 attenuates allodynic responses following CCI of the sciatic nerve, a well-defined model of neuropathic pain (Bennet and Xie, 1988). The mechanism underlying this effect is far from being clear. As in human neuropathies, the allodynic responses resulting from CCI largely depend on neurogenic inflammation distal to the site of the lesion (Daemen et al., 1998). Among other relevant factors, the expression of COX-2 and the release of peroxynitrite (Khalil and Khodr, 2001; Guedes et al., 2006), as well as inflammatory mediators including TNFα and IL-β (Poole et al., 1999), have been reported. Indeed, antioxidant agents modulate nociceptive transmission following chemically induced inflammatory pain (Crisp et al., 2006) and promote the anti-hyperalgesic activity of common NSAIDs (Alique et al., 2006). Furthermore, COX inhibition or exposure to PGE2 antibodies reverses peroxynitrite-induced inflammatory hyperalgesia (Ndengele et al., 2008). NCX 2057 retains the antioxidant activity of parent FA (Wenk et al., 2004) but, differently from FA, inhibits pro-inflammatory mediators in vitro (Ronchetti et al., 2006), exerts anti-inflammatory activity in vivo (Wenk et al., 2004) and exhibits COX inhibition in vitro and after carrageenan-induced paw inflammation (present study). Thus, the concomitant antioxidant and COX inhibitory activity of NCX 2057 in discrete peripheral and central sites might account for the profound antinociceptive features of our prototype drug. Here, we only addressed the acute effects of NCX 2057 and thus, it seems unlikely that inhibition of pro-inflammatory protein expression could have played a major role. Despite that, however, while COX-2 protein expression was unchanged following in vitro exposure to NCX 2057, iNOS expression and function were reduced dramatically in vitro, suggesting that NCX 2057-mediated gene regulation of some pro-inflammatory proteins may not be excluded at this time.
In conclusion, the results from the present study clearly document an intrinsic COX inhibitory activity for NCX 2057 that is not shared by FA. Given together the data obtained in previous work as well as in the current study, the observed anti-nociceptive/anti-inflammatory and anti-hyperalgesic effects could be ascribed to the antioxidant, COX inhibitory and NO-releasing activities of NCX 2057. Extending previous studies, the results from the present work further document the profile of NCX 2057 and would suggest a wide therapeutic application of this compound in the treatment of pain syndromes with different aetiologies.
Acknowledgments
This work was supported by EU Grant LSHM-CT-2004-005033 EICOSANOX and by a grant from the Spanish Ministry for Science and Technology (grant SAF2005-06242-C03-03).
Glossary
Abbreviations:
- AA
arachidonic acid
- CCI
chronic constriction injury
- COX-1
cyclooxygenase type-1
- COX-2
cyclooxygenase type-2
- Ferulic acid (FA
3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid
- IFNγ
interferon-γ
- LPS
lipopolysaccharide from Escherichia coli
- NCX 2057
3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid 4-(nitrooxy)butyl ester
- PGE2
prostaglandin E2
- PWT
paw withdrawal threshold
- SMU
single motor unit
Statement of conflicts of interest
Some of the authors (D.R., V.B. and F.I.) are employees of NicOx Research Institute S.r.l. and hold stock in NicOx.
References
- Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;2:79–84. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Alique M, Lucio FJ, Herrero JF. Vitamin A active metabolite, all-trans retinoic acid, induces spinal cord sensitization. II. Effects after intrathecal administration. Br J Pharmacol. 2006;149:65–72. doi: 10.1038/sj.bjp.0706826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Burian M, Geisslinger G. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol Ther. 2005;107:139–154. doi: 10.1016/j.pharmthera.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Crisp T, Minus TO, Coleman ML, Giles JR, Cibula C, Finnerty EP. Aging, peripheral nerve injury and nociception: effects of the antioxidant 16-desmethyltirilazad. Behav Brain Res. 2006;166:159–165. doi: 10.1016/j.bbr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Daemen MA, Kurvers HA, Kitslaar PJ, Slaaf DW, Bullens PH, Van den Wildenberg FA. Neurogenic inflammation in an animal model of neuropathic pain. Neurol Res. 1998;20:41–45. doi: 10.1080/01616412.1998.11740483. [DOI] [PubMed] [Google Scholar]
- Dong WG, Mei Q, Yu JP, Xu JM, Xiang L, Xu Y. Effects of melatonin on the expression of iNOS and COX-2 in rat models of colitis. World J Gastroenterol. 2003;9:1307–1311. doi: 10.3748/wjg.v9.i6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durate ID, Lorenzetti BB, Ferreira SH. Peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Eur J Pharmacol. 1990;186:289–293. doi: 10.1016/0014-2999(90)90446-d. [DOI] [PubMed] [Google Scholar]
- Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni M, Casagrande S, Maucci R, Chiroli V, Tocchetti P. In vitro metabolism of (nitrooxy)butyl ester nitric oxide-releasing compounds: comparison with glyceryl trinitrate. J Pharmacol Exp Ther. 2006;317:752–761. doi: 10.1124/jpet.105.097469. [DOI] [PubMed] [Google Scholar]
- Guay J, Bateman K, Gordon R, Mancini J, Riendeau D. Carrageenan-induced paw edema in rat elicits a predominant prostaglandin E2 (PGE2) response in the central nervous system associated with the induction of microsomal PGE2 synthase-1. J Biol Chem. 2004;279:24866–24872. doi: 10.1074/jbc.M403106200. [DOI] [PubMed] [Google Scholar]
- Guedes RP, Bosco LD, Teixeira CM, Araujo AS, Llesuy S, Bello-Klein A, et al. Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res. 2006;31:603–609. doi: 10.1007/s11064-006-9058-2. [DOI] [PubMed] [Google Scholar]
- Hancock CM, Riegger-Krugh C. Modulation of pain in osteoarthritis: the role of nitric oxide. Clin J Pain. 2008;24:353–365. doi: 10.1097/AJP.0b013e31815e5418. [DOI] [PubMed] [Google Scholar]
- Hara S, Kuhns ER, Ellenberger EA, Mueller JL, Shibuya T, Endo T, et al. Involvement of nitric oxide in intracerebroventricular beta-endorphin-induced neuronal release of methionine-enkephalin. Brain Res. 1995;675:190–194. doi: 10.1016/0006-8993(95)00065-x. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Cervero F. Changes in nociceptive reflex facilitation during carrageenan-induced arthritis. Brain Res. 1996;717:62–68. doi: 10.1016/0006-8993(95)01585-x. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Headley PM. The effects of sham and full spinalization on the systemic potency of µ- and k-opioids on spinal nociceptive reflexes in rats. Br J Pharmacol. 1991;104:166–170. doi: 10.1111/j.1476-5381.1991.tb12402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann C. COX-2 in brain and spinal cord implications for therapeutic use. Curr Med Chem. 2000;7:1113–1120. doi: 10.2174/0929867003374282. [DOI] [PubMed] [Google Scholar]
- Hosoda A, Ozaki Y, Kashiwada A, Mutoh M, Wakabayashi K, Mizuno K, et al. Syntheses of ferulic acid derivatives and their suppressive effects on cyclooxygenase-2 promoter activity. Bioorg Med Chem. 2002;10:1189–1196. doi: 10.1016/s0968-0896(01)00386-8. [DOI] [PubMed] [Google Scholar]
- Ibuki T, Matsumura K, Yamazaki Y, Nozaki T, Tanaka Y, Kobayashi S. Cyclooxygenase-2 is induced in the endothelial cells throughout the central nervous system during carrageenan-induced hind paw inflammation; its possible role in hyperalgesia. J Neurochem. 2003;86:318–328. doi: 10.1046/j.1471-4159.2003.01848.x. [DOI] [PubMed] [Google Scholar]
- Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil-induced peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Brain Res. 2001;909:170–178. doi: 10.1016/s0006-8993(01)02673-7. [DOI] [PubMed] [Google Scholar]
- Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med. 2001;31:430–439. doi: 10.1016/s0891-5849(01)00597-4. [DOI] [PubMed] [Google Scholar]
- Kean WF, Buchanan WW. The use of NSAIDs in rheumatic disorders 2005: a global perspective. Inflammopharmacology. 2005;13:343–370. doi: 10.1163/156856005774415565. [DOI] [PubMed] [Google Scholar]
- Lin Q, Wu J, Peng YB, Cui M, Willis WD. Inhibition of primate spinothalamic tract neurons by spinal glycine and GABA is modulated by guanosine 3′-5′-cyclic monophosphate guanosine. J Neurophysiol. 1999;81:1093–1103. doi: 10.1152/jn.1999.81.3.1095. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Tegeder I, Euchenhofer C, deWitt D, Brune K, Bang R, et al. Localization and regulation of cyclo-oxygenase-1 and -2 and neuronal nitric oxide synthase in mouse spinal cord. Neurosci. 2000;101:1093–1108. doi: 10.1016/s0306-4522(00)00361-4. [DOI] [PubMed] [Google Scholar]
- Ndengele MM, Cuzzocrea S, Esposito E, Mazzon E, Di Paola R, Matuschak GM, et al. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. FASEB J. 2008;22:3154–3164. doi: 10.1096/fj.08-108159. [DOI] [PubMed] [Google Scholar]
- Ongini E, Impagnatiello F, Bonazzi A, Guzzetta M, Govoni M, Monopoli A, et al. Nitric oxide (NO)-releasing statin derivatives, a class of drugs showing enhanced antiproliferative and antiinflammatory properties. Proc Natl Acad Sci USA. 2004;101:8497–8502. doi: 10.1073/pnas.0401996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrignani P, Tacconelli S, Sciulli MG, Capone ML. New insights into COX-2 biology and inhibition. Brain Res Brain Res Rev. 2005;48:352–359. doi: 10.1016/j.brainresrev.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Poole S, Lorenzetti BB, Cunha JM, Cunha FQ, Ferreira SH. Bradykinin B1 and B2 receptors, tumour necrosis factor alpha and inflammatory hyperalgesia. Br J Pharmacol. 1999;126:649–656. doi: 10.1038/sj.bjp.0702347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, et al. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinold H, Ahmadi S, Depner UB, Layh B, Heindl C, Hamza M, et al. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J Clin Invest. 2005;115:673–679. doi: 10.1172/JCI200523618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sandoval EA, Alique M, Moreno-Manzano V, Molina C, Lucio FJ, Herrero JF. The oral administration of retinoic acid enhances nociceptive withdrawal reflexes in rats with soft-tissue inflammation. Inflamm Res. 2004;53:297–303. doi: 10.1007/s00011-004-1261-5. [DOI] [PubMed] [Google Scholar]
- Ronchetti D, Impagnatiello F, Guzzetta M, Gasparini L, Borgatti M, Gambari R, et al. Modulation of iNOS expression by a nitric oxide-releasing derivative of the natural antioxidant ferulic acid in activated RAW 264.7 macrophages. Eur J Pharmacol. 2006;532:162–169. doi: 10.1016/j.ejphar.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Seybold VS, Jia YP, Abrahams LG. Cyclo-oxygenase-2 contributes to central sensitization in rats with peripheral inflammation. Pain. 2003;105:47–55. doi: 10.1016/s0304-3959(03)00254-9. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kulkarni SK, Chopra K. Resveratrol, a polyphenolic phytoalexin attenuates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rats. Indian J Exp Biol. 2006;44:566–569. [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Solano RE, Herrero JF. Cutaneous responsiveness of rat single motor units activated by natural stimulation. J Neurosci Methods. 1997;73:135–140. doi: 10.1016/s0165-0270(96)02220-0. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Yaksh TL. The spinal phospholipase-cyclooxygenase-prostanoid cascade in nociceptive processing. Annu Rev Pharmacol Toxicol. 2002;42:553–583. doi: 10.1146/annurev.pharmtox.42.092401.143905. [DOI] [PubMed] [Google Scholar]
- Tankova T, Cherninkova S, Koev D. Treatment for diabetic mononeuropathy with alpha-lipoic acid. Int J Clin Pract. 2005;59:645–650. doi: 10.1111/j.1742-1241.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- Valsecchi AE, Franchi S, Panerai AE, Sacerdote P, Trovato AE, Colleoni M. Genistein, a natural phytoestrogen from soy, relieves neurophatic pain following chronic constriction sciatic nerve injury in mice: anti-inflammatory and antioxidant activity. J Neurochem. 2008;107:230–240. doi: 10.1111/j.1471-4159.2008.05614.x. [DOI] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Warner TD, Mitchell JA. Cyclooxygenase-3 (COX-3): filling in the gaps towards a COX continuum? Proc Natl Acad Sci USA. 2002;99:13371–13373. doi: 10.1073/pnas.222543099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, McGann-Gramling K, Hauss-Wegrzyniak B, Ronchetti D, Maucci R, Rosi S, et al. Attenuation of chronic neuroinflammation by a nitric oxide-releasing derivative of the antioxidant ferulic acid. J Neurochem. 2004;89:484–493. doi: 10.1111/j.1471-4159.2004.02359.x. [DOI] [PubMed] [Google Scholar]
- Willingale HL, Gardiner NJ, McLymont N, Giblett S, Grubb BD. Prostanoids synthesized by cyclo-oxygenase isoforms in rat spinal cord and their contribution to the development of neuronal hyperexcitability. Br J Pharmacol. 1997;122:1593–1604. doi: 10.1038/sj.bjp.0701548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WP, Hao JX, Ongini E, Impagnatiello F, Presotto C, Wiesenfeld-Hallin Z, et al. A nitric oxide (NO)-releasing derivative of gabapentin, NCX 8001, alleviates neuropathic pain-like behavior after spinal cord and peripheral nerve injury. Br J Pharmacol. 2004;141:65–74. doi: 10.1038/sj.bjp.0705596. [DOI] [PMC free article] [PubMed] [Google Scholar]


