Abstract
Owing to the multifaceted functions of the large conductance Ca2+-activated K+ channel (BK), identification of protein-protein interactions is essential in determining BK regulation. A yeast two-hybrid screening of a cochlear cDNA library revealed a BK-ApoA1 interaction. Patch clamp recordings of excised membrane patches from transfected HEK293 cells showed that ApoA1 inhibits the BK α-subunit by significantly increasing activation and deactivation times, and shifting half-activation voltage to more positive potentials. Reciprocal coimmunoprecipitations verified the BK-ApoA1 interaction using excised sensory epithelium and ganglia. Additionally, immunocolocalization studies revealed BK and ApoA1 expression in both receptor cells and auditory neurons. These data suggest new avenues of investigation, given the importance of apolipoproteins in neurological diseases.
Keywords: Ion channels, Large-conductance calcium-activated potassium channels, Apolipoprotein, Protein-protein interactions, Cochlea, Hair cells
Ion channels have various roles in a number of different cell types and thus may encounter many different protein partners. The function of the BK channel is especially multifaceted, as it plays a role not only in excitation but also in intracellular signaling and metabolism. This functional complexity is reflected in findings that show BK expression in different subcellular compartments, including the mitochondrion and nuclear envelope [1-3]. BK will likely interact with a variety of proteins that are indigenous to these compartments and potentially alter cellular homeostasis [3].
Apolipoproteins have a significant role in various neurological diseases such as Alzheimer's, where they are found in both senile plaques and neurofibrillary fibers, and Creutzfeldt-Jakob disease, where they are found in kuru plaque amyloid [4]. This role extends to audition, since knockout mice, deficient in apolipoprotein E1 (ApoE1), lose high frequency hearing with the loss of receptor cells at the basal turn of the cochlea [5]. There are very few reported studies of lipoprotein effects on channel activity, however, despite the prevalence of these proteins in neuronal systems. A recent report shows that ApoE4 alters the biophysical properties of delayed rectifier type channels when applied to excised patches from hippocampal neurons [6], whereas oxidized low density lipoproteins appear to affect the kinetics of BK channels in cultured endothelial cells [7].
In the present study, a yeast two hybrid screening of a cDNA library made from embryonic days 14-19 chicken cochlea revealed an interaction between BK and the apolipoprotein, ApoA1, a component of high density lipoproteins. Here, we provide functional evidence for this interaction in excised membrane patches, when coexpressed in HEK293 cells. Moreover, we demonstrate that ApoA1 and BK are coexpressed in both neuronal and receptor cells. To date, apolipoprotein effects on ion channels have used acute application techniques. Thus, to the best of our knowledge, this study is the first to show a direct apolipoprotein-ion channel association via cellular expression.
Materials and methods
Yeast two-hybrid screening
The full BK channel inserted into pBD-Gal4 Cam (Stratagene) at the EcoRI and SalI restriction sites was used as bait to screen a cDNA library made from embryonic days 14-19 Gallus gallus sensory epithelium from the basilar papilla (i.e., cochlea) as described previously [8]. Yeast AH109 cells (Clontech) were transformed sequentially, first with pBDGal-4 containing the bait construct and then with pADGal-4/cDNA library using the lithium acetate/single-stranded carrier DNA/polyethylene glycol method [9]. Cells were plated on SD-His-Leu-Ade-Trp (SD-HLAT) medium and positive colonies selected and analyzed. Full-length cDNA fragments of ApoA1 were obtained and subcloned into the pCMS-EGFP vector (BD Bioscience) for co-expression experiments, to study the biophysical effects on the BK channel.
Electrophysiology
Heterologous expression and biophysical characterization of BK channel constructs were performed as described previously [10]. BK channels, with and without ApoA1, were transiently expressed in HEK293T cells using standard Ca2+-phosphate transfection techniques. HEK cells were maintained in a high-glucose DMEM with 10% fetal bovine serum and 1% penicillin/streptomycin and cultured at 37 °C. To limit genetic drift, cells were passaged no more than 20 times. Plasmid constructs included the minimal variant of the chicken BK α-subunit (cslo1) in a mammalian expression vector (pcDNA3.1) [10, 11] and ApoA1 in pCMS-EGFP. When required, ApoA1 was added at two-fold molar excess to BKα in order to saturate potential stoichiometric interactions between these proteins. Transfections using BKα alone included pEGFPLuc (BD Biosciences Clontech) for fluorescence detection of transfected cells.
Patch clamp recordings were obtained from excised membrane patches in the inside-out configuration [12]. Cells were obtained 24 to 48 h after transfection. Bath solutions, pipette solutions, and perfusate each consisted of a high K+ saline with (mM): 142 KCl, 0.5 MgCl2, and 5 HEPES at pH 7.2. CaCl2 and a suitable buffer were added to achieve desired free Ca2+ concentrations. All solutions were adjusted to a pH of 7.2 using KOH. After excision, the inside face of the patch was exposed to a high K+ perfusate calibrated to contain free Ca2+ concentrations of 1 or 20 μM using the Ca2+ chelators Br2BAPTA or NTA, respectively [13]. The pipette solution was identical to the 1 μM free Ca2+ test solution in order to set the K+ reversal potential to 0 mV and minimize junction potentials. Ca2+ concentration was calibrated using a Ca2+ -sensitive electrode and multiple Ca2+ standards (WPI, Sarasota, FL).
Microelectrodes were pulled from borosilicate glass to resistances of 5-9 MΩ and coated with R6101 (Dow-Corning) to reduce stray capacitance. Recordings were made with a MultiClamp 700B amplifier, Digidata 1440A digitizer, and the pClamp 10.0 software suite (Molecular Devices, Sunnyvale, CA). Data were sampled at 10 to 20 kHz, and low-pass filtered at 4 kHz. Each patch was estimated to contain between 4 - 160 BK channels, assuming a single channel conductance of 250 pS and a maximum open probability near unity. Transient and steady-state BK properties were determined from ensemble-averaged current recordings of 5 to 20 stimulus presentations. Pre-pulses to −50 or −100 mV were used to deactivate the channels prior to activation steps and ascertain the linear leak currents, which were subtracted off-line. Residual capacitance currents at the K+ equilibrium potential were fit with exponentials and piecewise linearly subtracted off-line to resolve fast deactivation kinetics.
Statistical analyses of individual pairs were performed using a Student's t-test, whereas multiple group comparisons were analyzed using an ANOVA. Activation and deactivation kinetics were estimated with least square fits to standard single-exponential curves in Clampfit (pClamp) using a Levenberg-Marquardt algorithm. Averaged values are reported as the mean ± standard error of the mean (S.E.M.) with significance indicated by p < 0.05. All recordings were made at room temperature (22-25°C).
Coimmunoprecipitation
Immunoprecipitation was accomplished using the immunocomplex-capture technique. Eight cochleae from 3 week-old chickens, including sensory epithelium, ganglion and supporting cells were removed and processed as described previously [14, 15]. The sample was centrifuged and the pellet solubilized in lysis buffer containing 50 mM Tris-HCl, pH 8.0, 120 mM NaCl, 5 mM EDTA, 50 mM NaF, 500 μg/ml AEBSF, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, 2 μg/ml aprotinin, 5 μM okadaic acid, and 0.1% ASB-14 (Calbiochem), followed by vortexing and agitating on a rocker for 1 h at 4°C. The fraction was precleared using rec-Protein G Sepharose 4B beads (Invitrogen). Monoclonal anti-BK antibody (5μg) (BD Biosciences; aa residues 995 – 1113 of human KCNMA1) was added to the sample and incubated at 4°C for 1 h followed by immunocomplex capture using 25 μl of protein G beads at 4°C. Beads were washed in lysis buffer, eluted in sample buffer (Sigma), and heated at 70°C. Samples were fractionated on a 10% SDS-PAGE gel then blotted, followed by blocking in PBST, containing 5% milk for 1 h. Blots were probed with either the monoclonal anti-BKα antibody (1:200; BD Biosciences) or a polyclonal anti-ApoA1 antibody (1:300; Abcam). HRP-conjugated donkey anti-rabbit or goat anti-mouse secondary antibodies were used for visualization of both ApoA1 (1:5000) and BKα (1:4000), respectively. Immunoreactive bands were developed using ECL (Amersham). Controls consisted of sample incubation with beads alone and IPs with antibodies. Antigens for preadsorption controls were not available.
Fluorescent Confocal Microscopy
Colocalization of BK and ApoA1 was determined in receptor and ganglion cells using respective antibodies with frozen sections prepared as described previously [16]. Briefly, three-week old chick basilar papillae (cochlea) with lagena were dissected partially from the temporal bone and fixed overnight (O/N) at 4°C by immersion in 4% paraformaldehyde in 100 mM phosphate buffer, pH 7.4. The remaining temporal bone was removed the next day and the tissues immersed in 30% sucrose O/N, and infiltrated in a 1:1 mixture of O.C.T. (Sakura Fine-Tek) and 30% sucrose under vacuum for 1 hr. Cryosections of 20 μm thickness were collected and permeabilized with 0.3 % Triton X-100 followed by blocking with 10% goat serum. Primary antibodies consisted of a monoclonal anti-BKα antibody (1:7.5; Antibodies Inc.) and a polyclonal anti-ApoA1 antibody (1:4; Abcam). Immunoreactivity was visualized using secondary antibodies labeled with the chromogens AlexaFluor 488 (ApoA1) and AlexaFluor 594 (BK) (Invitrogen). Sections were imaged with a Leica SP5 AOBS tandem scanning inverted confocal microscope (Lasos, San Jose, CA) at the Microscopy Core Facility, Moffitt Cancer Center, and Z-stacks were acquired using a step size of 0.2 μm.
Results and discussion
ApoA1 affects BK channel characteristics
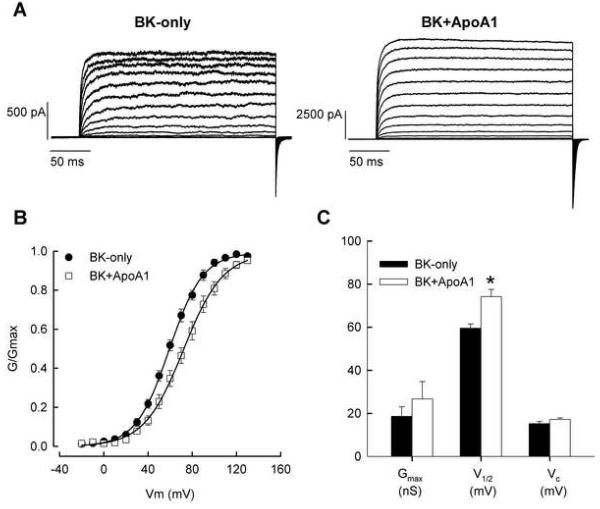
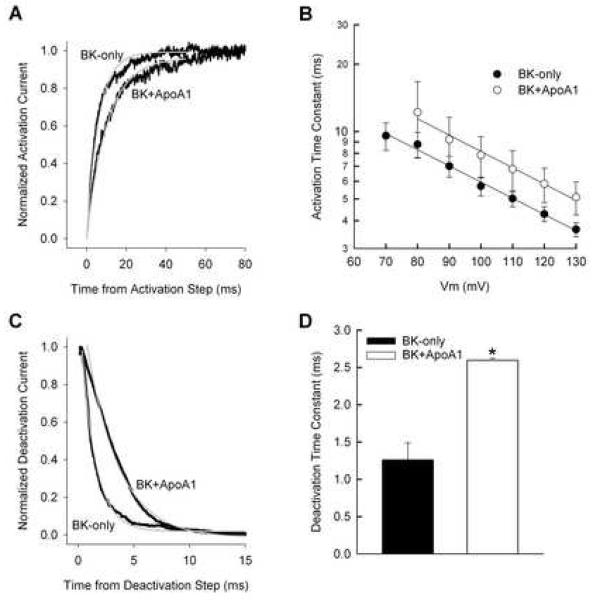
A Y2H screening using pBD-Gal-4/BK as bait resulted in yeast colonies containing full-length Gallus ApoA1 (Acc. No. M18746). Patch clamp analyses showed that ApoA1 inhibited the channel kinetics of BKα (Fig. 1). In the presence of ApoA1, the BK steady-state conductance-voltage (G-V) curve shifted to more positive potentials by ∼11.6 mV. Moreover, in the presence of 1 μM Ca2+, the half-activation voltage for BK channels increased, showing mean values, with and without ApoA1, of 69.8 ± 4.5 mV (n = 9) and 58.2 ± 1.6 mV (n = 8; p < 0.05), respectively. The inhibitory effects of ApoA1 were seen again when measuring the activation and deactivation times of BKα, in the presence of 1 μM Ca2+ (Fig. 2). As expected, both in the presence and absence of ApoA1, activation time constants decreased significantly with increased membrane potential from 80 to 130 mV (ANOVA; FMEMB. POTENTIAL = 2.9, df = 6, p < 0.05). However, ApoA1 increased activation time constants by ∼3 ms at these potentials (ANOVA; FTREATMENT = 4.1, df, = 1, p < 0.05). BKα deactivation responded similarly in the presence of ApoA1, showing a significant increase in the time constant from 1.3 ± 0.2 ms (n = 4) to 2.6 ± 0.3 ms (n=9; p < 0.5). In contrast, ApoA1 had no statistically significant effect on Gmax, and the Boltzman slope factor (Vc). Gmax values measured for cells transfected with and without ApoA1 were 21.6 ± 10.6 nS (n = 9) and 16.9 ± 4.8 nS (n = 8; p > 0.05), respectively, whereas Vc measured 17.1 +/− 0.77 mV and 15.2 +/− 1.0 mV (p > 0.05).
Fig. 1.
ApoA1 increases BK half-activation voltage. (A) Currents recorded from isolated inside-out patches of HEK293 cells transfected with BK only or BK+ApoA1. Patches were stepped from −20 mV to 130 mV from a holding potential of −50 mV. Tail currents were generated by a final step back to −50 mV. (B) Average, normalized G-V curves were generated for patches containing BK-only (n = 8) and BK+ApoA1 (n = 9). Individual G-V curves were fit to Boltzmann equations and fit parameters averaged. (C) Plots of the average Gmax, half-activation voltage (V1/2), and Boltzman slope factor (Vc) are shown for the two-channel configurations. (*p < 0.05).
Fig. 2.
ApoA1 slows BK kinetics. (A) Exemplar of activation currents for a voltage step from −50 mV to 100 mV. Traces were normalized to steady-state maxima after correcting for linear leak and capacitative transients. Single exponential fits (grey lines) are 6.03 and 10.32 ms for BK only and BK+ApoA1, respectively. (B) Average activation time constants plotted against the activation voltage command and fit with a single exponential show an ∼3 ms shift in BK activation with ApoA1 present. (C) Exemplar deactivation currents for a voltage step from 130 mV to −50 mV. Traces were normalized and fit as in A. Single exponential fits (grey lines) are 1.29 and 2.85 ms for BK only and BK+ApoA1, respectively. (D) Deactivation kinetics at a single voltage level of −50 mV were averaged from single exponential fits to tail currents elicited from a 130 mV activation step. Deactivation increased ∼2-fold with ApoA1. Error bars indicate one SEM. (*p < 0.05)
These results demonstrate that ApoA1 has an inhibitory effect on BK response properties at the membrane as opposed to acting as a chaperone that increases channel density. This inhibitory effect would alter the hyperpolarization characteristics of excitatory cells and more specifically, in hair cells, the tuning properties. Chick hair cells, as those in turtle [17, 18] show receptor potential oscillations or electrical “tuning.” This resonance is a function of BK channel number and kinetics [19], which may be varied systematically along the cochlea by several mechanisms including splice variation and co-assembly with accessory proteins. Thus, with ApoA1 inhibition, one might expect a decrease in the resonant frequency or tuning of the receptor cell.
The BK channel is sensitive to Ca2+ concentrations, exhibiting as many as three Ca2+-binding sites [20]. To test the possibility that inhibition by ApoA1 was due to a change in Ca2+ sensitivity, the effect of ApoA1 was tested in a higher Ca2+-free concentration. When exposed to 20 μM [Ca2+]i, the half-activation voltage for channels with and without ApoA1 was −1.4 ± 3.4 mV and 10.7 ± 2.6 mV (p < 0.05, n = 5), respectively (G-V data not shown). This shift in V1/2 of ∼11.1 mV was similar to the shift measured when using 1 μM [Ca2+]i, suggesting that ApoA1 influences channel gating by a Ca2+-independent mechanism.
Regulators of BK can be both Ca2+-independent and dependent. For example, the addition of purified PKCδ activates BK channels, and alters the biophysical characteristics, as V1/2 decreases with increased Ca2+ concentrations [21]. Similarly, syntaxin 1A has a Ca2+-dependent effect by increasing BK activity at low Ca2+ concentrations (1-4 μM), but not at high Ca2+ concentrations (>10 μM) [22]. In contrast, the lipid, N-arachidonoyl L-serine, and the xenoestrogen, tamoxifen, show no Ca2+ dependency [23, 24].
ApoA1 is expressed in sensory and ganglion cells
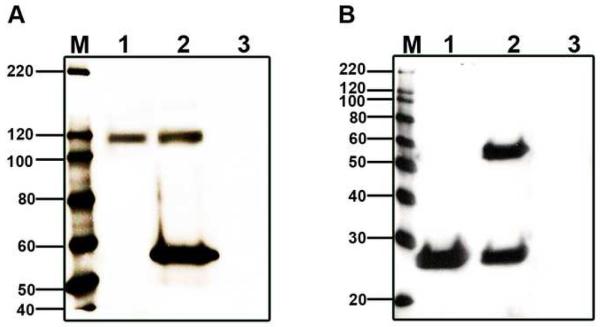
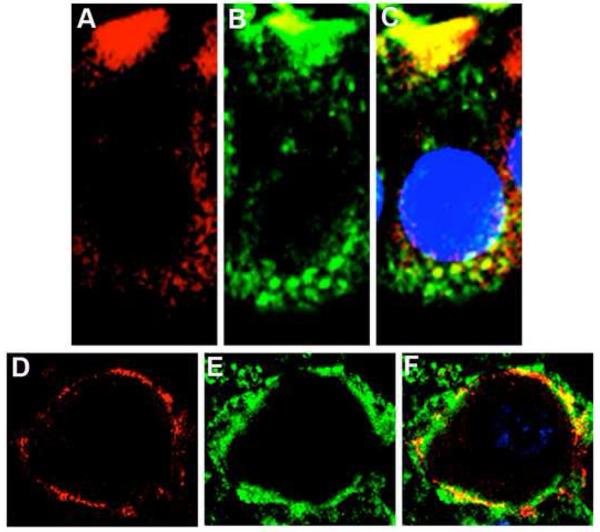
Reciprocal coIPs, using whole cochlea, demonstrate that BK and ApoA1 interact in the cochlea, since each protein was able to immunoprecipitate the other (Fig. 3). ApoA1 immunoprecipitated BKα, which is seen as an ∼120 kDa band. Similarly, BK immunoprecipitated ApoA1 as observed by the band at ∼28 kDa (Fig. 3). Colocalization studies using fluorescent tags, showed that BKα and ApoA1 are colocalized in receptor and ganglion cells (Fig. 4). Previous studies of apolipoproteins in cochlea were restricted to ApoE1 and ApoD, where mice deficient in the former showed a hearing loss and substantial cochlear cell death [5, 25], while ApoD knockouts had normal hearing thresholds [26]. Putative roles include cochlear ionic and oxidative homeostasis [25, 26], a balance that may be connected to ion channels.
Fig. 3.
Verification of BK/ApoA1 interactions using reciprocal coIP. (A) CoIP of BKα with ApoA1 (lane 1) and IP of BKα (lane 2) reveal prominent bands at ∼120 kDa, the expected weight of chick BKα. (B) Reciprocal coIP of ApoA1 with BKα (lane 1) and IP of ApoA1 alone (lane 2) reveal a prominent band at ∼28 kDa, the expected weight of ApoA1. The control for both panels consisted of mixing IgG beads and lysate in the absence of an antibody (lane 3). The 55 kDa band is heavy IgG, and occurs when either monoclonal or polyclonal antibodies are used to both precipitate proteins and probe the blots. “M” denotes the standard.
Fig. 4.
ApoA1 (green) and BKα (red) are colocalized in chick cochlea. The colocalization of ApoA1 and BK is visualized as punctate yellow fluorescence in (A) basolateral aspects of hair cells and (B) VIIIth nerve ganglion cell body. Images are from one section of a Z-stack acquired using a step size of 0.2 μm.
In summary, we demonstrated the effectiveness of ApoA1 in altering the kinetics of the BK channel. Previously, acute application of ApoE1, a component of intermediate-density lipoproteins, changed the kinetics of the delayed rectifier channel in hippocampal cells [6]. Interestingly, ApoA1 had no effect on this channel despite its similarity in weight and amino acid sequence to ApoE [6]. These discrepancies may be the result of methodologies or physical differences in the channels. Nonetheless, given the different types of apolipoproteins, their association with ion channels should be explored further in relation to neurological diseases.
Acknowledgments
We thank Dr. S. Heller for the pADGal4-cDNA library made from cochlear sensory epithelium. B. S. was supported by NIH/NIDCD grant R01 DC004295 and R. K. D. by NIH/NIDCD grant R01 DC007432 and grant P30 DC0578188 to the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maruyama Y, Shimada H, Taniguchi J. Ca(2+)-activated K(+)-channels in the nuclear envelope isolated from single pancreatic acinar cells. Pflügers Arch. 1995;430:148–150. doi: 10.1007/BF00373851. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita M, Sugioka M, Ogawa Y. Voltage- and Ca2+-activated potassium channels in Ca2+ store control Ca2+ release. FEBS J. 2006;273:3585–3597. doi: 10.1111/j.1742-4658.2006.05365.x. [DOI] [PubMed] [Google Scholar]
- 3.O'Rourke B. Mitochondrial ion channels. Ann Rev Physiol. 2007;69:19–49. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y, Zhang C, Du X, Nair U, Yoo TJ. Morphological and functional alterations of the cochlea in apolipoprotein E gene deficient mice. Hear Res. 2005;208:54–67. doi: 10.1016/j.heares.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Qin Y, Qi JS, Qiao JT. Apolipoprotein E4 suppresses delayed-rectifier potassium channels in membrane patches excised from hippocampal neurons. Synapse. 2006;59:82–91. doi: 10.1002/syn.20201. [DOI] [PubMed] [Google Scholar]
- 7.Kuhlmann CR, Schäfer M, Li F, Sawamura T, Tillmanns H, Waldecker B, Wiecha J. Modulation of endothelial Ca(2+)-activated K(+) channels by oxidized LDL and its contribution to endothelial proliferation. Cardiovasc Res. 2003;60:626–634. doi: 10.1016/j.cardiores.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Duzhyy D, Harvey M, Sokolowski B. A secretory-type protein, containing a pentraxin domain, interacts with an A-type K+ channel. J. Biol. Chem. 2005;280(15):15165–15172. doi: 10.1074/jbc.M500111200. [DOI] [PubMed] [Google Scholar]
- 9.Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrierDNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 10.Jiang GJ, Zidanic M, Michaels RL, Michael TH, Griguer C, Fuchs PA. CSlo encodes calcium-activated potassium channels in the chick's cochlea. Proc. Biol. Sci. 1997;264(1382):731–737. doi: 10.1098/rspb.1997.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan RK. Tamoxifen alters gating of the BK alpha subunit and mediates enhanced interactions with the avian beta subunit. Biochem. Pharmacol. 2005;70(1):47–58. doi: 10.1016/j.bcp.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 12.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 13.Duncan RK, Fuchs PA. Variation in large-conductance, calcium-activated potassium channels from hair cells along the chicken basilar papilla. J. Physiol. 2003;547(2):357–371. doi: 10.1113/jphysiol.2002.029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kathiresan T, Harvey MC, Sokolowski BH. The use of 2-D gels to identify novel protein-protein interactions in the cochlea. In: Sokolowski B, editor. Protocols in Auditory Research. Humana Press/Springer Protocols; New York: 2009. pp. 269–286. [DOI] [PubMed] [Google Scholar]
- 15.Kathiresan T, Harvey M, Orchard S, Sakai Y, Sokolowski B. A protein interaction network for the large conductance Ca2+-activated K+ channel in themouse cochlea. Mol. Cell. Proteomics. 2009 May 7; doi: 10.1074/mcp.M800495-MCP200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokolowski BH, Sakai Y, Harvey MC, Duzhyy DE. Identification and localization of an arachidonic acid-sensitive potassium channel in the cochlea. J. Neurosci. 2004;24(28):6265–6276. doi: 10.1523/JNEUROSCI.1291-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford AC, Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J. Physiol. 1981;312:377–412. doi: 10.1113/jphysiol.1981.sp013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs PA, Nagai T, Evans MG. Electrical tuning in hair cells isolated from the chick cochlea. J. Neurosci. 1988;8(7):2460–2467. doi: 10.1523/JNEUROSCI.08-07-02460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Art JJ, Crawford AC, Fettiplace R. Electrical resonance and membrane currents in turtle cochlear hair cells. Hear. Res. 1986;22:31–36. doi: 10.1016/0378-5955(86)90073-0. [DOI] [PubMed] [Google Scholar]
- 20.Zeng XH, Xia XM, Lingle CJ. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J. Gen. Physiol. 2005;125(3):273–286. doi: 10.1085/jgp.200409239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JY, Park CS. Potentiation of large-conductance calcium-activated potassium (BK(Ca)) channels by a specific isoform of protein kinase C. Biochem. Biophys. Res. Commun. 2008;365(3):459–465. doi: 10.1016/j.bbrc.2007.10.179. [DOI] [PubMed] [Google Scholar]
- 22.Ling S, Sheng JZ, Braun JE, Braun AP. Syntaxin 1A co-associates with native rat brain and cloned large conductance, calcium-activated potassium channels in situ. J. Physiol. 2003;553:65–81. doi: 10.1113/jphysiol.2003.051631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dick GM, Rossow CF, Smirnov S, Horowitz B, Sanders KM. Tamoxifen activates smooth muscle BK channels through the regulatory beta 1 subunit. J Biol Chem. 2001;276(37):34594–34599. doi: 10.1074/jbc.M104689200. [DOI] [PubMed] [Google Scholar]
- 24.Godlewski G, Offertáler L, Osei-Hyiaman D, Mo FM, Harvey-White J, et al. The endogenous brain constituent N-arachidonoyl L-serine is an activator of large conductance Ca2+-activated K+ channels. J Pharmacol Exp Ther. 2009;328(1):351–361. doi: 10.1124/jpet.108.144717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu S, Du X, Cai Q, Guo Y, Liu L, Cheng W, Tao Z, Yoo T. Impaired stria vascularis in the inner ear of apolipoprotein E gene knockout mice. ORL J. Otorhinolaryngol. Relat. Spec. 2008;70(6):373–380. doi: 10.1159/000163033. [DOI] [PubMed] [Google Scholar]
- 26.Hildebrand MS, de Silva MG, Klockars T, Solares CA, Hirose K, Smith JD, Patel SC, Dahl HH. Expression of the carrier protein apolipoprotein D in the mouse inner ear. Hear. Res. 2005;200(12):102–114. doi: 10.1016/j.heares.2004.08.018. [DOI] [PubMed] [Google Scholar]