Abstract
Enterocyte apoptosis in necrotizing enterocolitis is partly due the elaboration of toxic intermediates of nitric oxide (NO), such as peroxynitrite (PN). Because p38 mitogen-activated protein kinase (MAPK) and serine-threonine kinase (AKT) are well-characterized pro- and anti-apoptotic mediators respectively, we hypothesized that PN could induce enterocyte apoptosis via activation of p38 and deactivation of AKT. To test this hypothesis, the rat intestinal cell line, IEC-6, was treated with PN. PN caused phosphorylation of p38, its upstream activator, MKK3/6, and downstream effector, transcription factor ATF2. PN-induced apoptosis was inhibited by the p38 inhibitor, SB202190, and by p38 siRNA. PN decreased AKT phosphorylation; this effect was abrogated by pre-treatment with SB202190 or p38 siRNA. PN exposure also increased the activity of the protein phosphatase 2A (PP2A). These data demonstrate that PN-mediated apoptosis depends on the p38 pathway and that p38 mediates deactivation of AKT survival pathways possibly by the involvement of PP2A.
Keywords: necrotizing enterocolitis; nitric oxide; peroxynitrite, enterocytes; p38; AKT
INTRODUCTION
Necrotizing enterocolitis (NEC) is a devastating inflammation of the intestine seen primarily in pre-term neonates. Epithelial injury caused by perinatal insults such as formula feeding, abnormal bacterial colonization of the gut, hypoxia, and hypothermia leads to bacterial translocation across the epithelium [1]. Bacteria then stimulate sensory cells of the innate immune system such as macrophages and monocytes, as well as enterocytes, to produce proinflammatory cytokines/chemokines, and nitric oxide (NO)[2]. NO and its reactive oxidation intermediate, peroxynitrite (PN), play an important role in the pathogenesis of NEC [3]. Physiological levels of NO are relatively innocuous, and at low levels NO is protective by virtue of its ability to increase blood flow [4]. However, inflammatory up-regulation of inducible nitric oxide synthase (iNOS) during NEC leads to dramatic local accumulation of NO and formation of PN, which leads to epithelial injury [4].
Mechanisms leading to cellular injury due to high levels of NO and PN are complex. NO or its derivatives may induce conformational changes in iron-sulfur linkages within the catalytic domain of enzymes involved in the mitochondrial electron transport chain such as NADH: ubiquinone oxido-reductase, NADH: succinate oxido-reductase, and aconitase of the Krebs cycle [5]. In addition, NO may cause DNA damage directly through deamination reactions, or by increasing oxidative stress via formation of PN [6]. We previously demonstrated that intestinal injury in the rat model of NEC is associated with increased immunoreactivity to 3-nitrotyrosine, a molecular footprint of PN [7]. We also showed that PN can induce enterocyte apoptosis through a caspase-mediated pathway in the rat intestinal epithelial cell line IEC-6 [8]. Furthermore, PN decreased epithelial cell proliferation by inhibiting the Src kinase pathway, thus inhibiting tissue repair mechanisms[9].
The mitogen-activated protein kinase (MAPK) pathways regulate various aspects of inflammatory stress responses[10]. p38 is a member of the MAPK family that is activated by stressors and inflammatory factors[10]. p38 activation may lead to enterocyte death by up-regulating proteins responsible for apoptosis. An example of such a protein is tensin homolog deleted on chromosome ten (PTEN), which promotes apoptosis by inhibiting phosphorylation of the serine-threonine protein kinase AKT [11]. The phosphatidylinositol 3'-kinase (PI 3-K)/AKT pathway is critical for cell survival and prevention of apoptosis [12]. We hypothesized that PN-mediated enterocyte apoptosis is governed by the p38 MAPK and AKT pathways. In this study, we show that PN induces enterocyte apoptosis by inhibiting phosphorylation of AKT in a p38-dependent manner.
MATERIALS AND METHODS
Materials
The reagents used were from the following suppliers: SB202190, okadaic acid (OA), 7-amino-actinomycin D (7-AAD), and anti- -actin monoclonal antibody (Sigma-Aldrich, St. Louis, MO); anti-AKT, anti-phospho-AKT, anti-MKK3, anti-phospho-MKK3/6, anti-p38, anti-phosphop38, anti-activating transcription factor 2 (ATF2), and anti-phospho-ATF2 (Cell Signaling Technology, Beverly, MA); gliotoxin and protein phosphatase 2A Inhibitor I (I1 PP2A) (Calbiochem, Gibbstown, NJ).
Cell Culture
The rat intestinal IEC-6 cell line was purchased from American Type Culture Collection. Cells (passages 16-29) were grown at 37 °C and 5% CO2 to 70-90% confluence in Dulbeco's modified Eagle's medium with 4.5 g/l glucose supplemented with 5% FBS, 2 mM glutamine, 0.1 U/ml penicillin, and 100 g/ml streptomycin. Cells were treated with PN (Alexis, San Diego, CA) or DPN in PBS following two washes with PBS. DPN was prepared by diluting equivalent amount of PN in PBS and incubating the solution at room temperature overnight.
p38 MAPK siRNA
Rat p38 siRNA (sense:5'-GGACCUCCUUAUAGACGAAUU) [anti-sense: 5'-UUCGUCUAUAAGGAGGUCCUU) duplexes or control siRNA (5'-UAGCGACUAAACACAUCAAA) were transfected into IEC-6 cells using DharmaFect-4 solution (Dharmacon, Chicago, IL) using manufacturer's protocol. Transfected cells were allowed to recover for 48 h prior to experiments.
Western Blot Analyses
Cells were lysed in RIPA buffer (20mMTris-HCl, pH 7.4, 137 mM NaCl, 10% glycerol, 1% NP-40, 10 g/ml aprotinin, and 10 g/ml leupeptin). The supernatant was collected after centrifugation at 14000g for 10 min. Aliquots of protein (50 g) were electrophoresed on 4-15% gradient gels (Bio-Rad, Hercules, CA) using a mini-gel system and transferred to nitrocellulose membranes (GE, Minnetonka, MN). Membranes were blocked with 5% milk in PBS with 0.1% Tween 20 for 1h at room temperature and then incubated with primary and secondary horseradish peroxidase-conjugated antibodies as recommended by antibody manufacturers. Protein bands were visualized with Super Signal chemiluminescence substrate (Pierce, Rockford, IL).
Apoptosis Assay
IEC-6 cells were treated with PN, DPN or gliotoxin and incubated in growth medium for 6h after exposure. Cells were then trypsinized, stained with 4 g of 7-AAD in PBS, washed with PBS, and analyzed by flow cytometry as previously described [13].
Phosphatase Assay
Phosphatase activity was measured using Ser/Thr Phosphatase assay kit optimized to detect PP2A (Upstate Signaling, Lake Placed, NY). Cells were exposed to PN or DPN, with or without 30 min pre-treatment with the two inhibitors of PP2A, OA (1 M) or I1 PP2A (100nM), or the p38 inhibitor SB202190. Cells were lysed in phosphatase buffer (20mM imidazole-HCl, 2mM EDTA, 2mM EGTA, 10 g/mL of aprotinin, leupeptin, antipain, soybean trypsin inhibitor, 1mM of benzamidine and PMSF). Protein concentration was determined (Bio-Rad). After clearing by centrifugation at 2000xg for 5 min, supernatants were equilibrated to 50mM Tris-HCl, pH 7.0, 0.1mM CaCl2 on Sephadex G-25 columns (Roche, Indianapolis, IN). PP2A activity was determined using the kit (Upstate, Lake Placid, NY) as recommended by the manufacturer.
Statistical Analysis
The data were analyzed for statistical significance by Student's t-tests or ANOVA using the SigmaStat software package (Systat Software, San Jose, CA).
RESULTS
PN increases Phospho-p38 (P-p38),phospho-MKK3/6 and phosho-Activating Transcription Factor -2 (ATF-2) in vitro
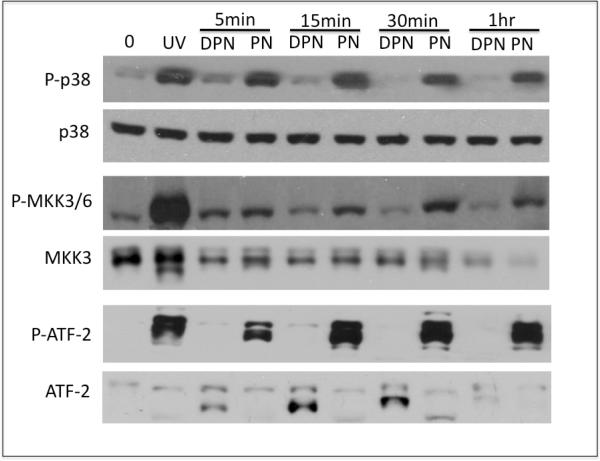
Exposure of IEC-cells to PN, but not DPN, caused time-dependent increase in the activating phosphorylation of p38 (Fig 1). The increase in p38 phosphorylation occurs as early as 5 min of exposure to PN, and persists up to 1h. MAPK kinases 3 and 6 (MKK3/6) are the known upstream activators of p38 [14; 15]. To determine whether MKK3/6 are activated by PN, we examined PN-induced activating phosphorylation of these kinases. As shown in figure 1, PN causes an increase in phospho-MKK3/6. PN exposure and persists for as long as 1 h, which is consistent with MKK3/6 mediating the PN-induced p38 phosphorylation. p38 is known to phosphorylate the transcription factor ATF-2, which, as part of the C/EBP transcription complex, activates transcriptional expression of the inflammatory stress response and pro-apoptotic genes, including Gadd-45 [16]. To test whether PN activates ATF-2, we examined levels of phospho-ATF-2 in PN-treated cells. PN, but not DPN, increased ATF-2 phosphorylation, persisting at least 1h (Fig 1). Our data show that PN is likely to activate ATF-2 via a canonical p38 cascade that involves MKK3/6.
Figure 1. PN increases phosphorylation of p38, MKK3/6 and ATF-2.
IEC-6 cells were exposed to 50 M PN and DPN for the indicated times. Naïve (untreated cells) are indicated by 0min. Levels of phospho-p38, phospho-MKK3/6, phospho-ATF-2, total p38, total MMK3 and total ATF-2 were determined by Western blotting. UV, cells exposed to 50mJ/cm2 UVC and allowed to recover in growth medium for 1h (positive control for p38 activation). Data shown are representative of at least 3 independent experiments.
PN-induced apoptosis is prevented by p38 inhibition
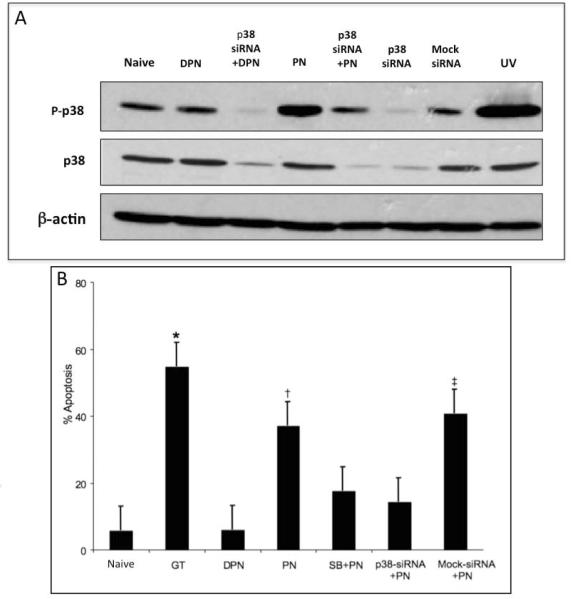
We first tested whether transfection with p38 siRNA could decrease the levels of p38 phosphorylation in response to PN exposure (Fig 2A). Cells transfected with p38 siRNA, but not with control (mock) siRNA, had decreased levels of p38 expression (Fig 2A). Moreover, siRNA knockdown of p38 expression caused a marked decrease in PN-induced phospho-p38 signal (Fig 2A). These results show that PN induces phosphorylation of p38, and that levels of phospho-p38 in PN-treated cells can be reduced by transfection with p38 siRNA (Fig 2A).
Figure 2. PN-induced apoptosis is prevented by p38 inhibition.
(A) IEC-6 cells were transfected with p38 siRNA, or control (Mock) siRNA as indicated. 36h post transfection, cells were exposed to PBS, or 50 M PN, or equivalent concentration of DPN for 60min as indicated, and levels of phospho-p38, total p38, and -actin were determined. UV, cells exposed to 50 mJ/cm2 UVC and allowed to recover in growth medium for 1h (positive control for p38 activation). (B) IEC-6 cells were treated with PN or equivalent concentration of DPN for 1h, and allowed to recover for 6h. Percentage of apoptotic (sub-G1 DNA content) cells was determined by flow cytometry of 7-AAD stained cells were determined by flow cytometry. Cells were pre-treated with 10 M SB202190 (SB), or transfected with p38 siRNA or control siRNA 30 min or 48h prior to PN or DPN treatment, respectively, as indicated. GT, cells treated with 2.5 mol/L of gliotoxin for 6h. (*) p<0.001, gliotoxin treated cells vs. naïve DPN-treated, SB+PN-treated or p38 siRNA-transfected cells. (†) p<0.001, PN-treated vs. DPN-treated, SB+PN-treated and p38 siRNA-transfected PN-treated cells. (‡) p<0.001 control-siRNA-transfected vs.p38-siRNA-transfectedPN-treated cells. Data are depicted as the mean S.E. (n=6).
To test whether p38 inhibition could prevent PN-induced enterocytes apoptosis, we determined the percentage of apoptotic cells following exposure to PN. Apoptotic cells were identified as cells with sub-G1 DNA content by flow cytometry following staining with 7-AAD (Fig 2B). Naïve cells had a baseline mean apoptosis rate of 5.6% 2.9, compared to 36.8% 4.4 in PN treated cells (p=0.001, n=6). As expected, exposure to PN, but not to DPN (5.9% 2.6), increased the percentage of apoptotic cells (p<0.001, n=6). There was no difference between DPN and naïve cells in terms of mean apoptosis (p=0.9, n=6). Gliotoxin is potent an inducer of enterocyte apoptosis (positive control) [13]. Gliotoxin caused apoptosis in 54% 2.3 cells. Cells pretreated with the p38 inhibitor, SB202190, and exposed to PN (17.5% 0.5) had a two-fold decrease in levels of apoptosis compared to cells treated with PN alone (p<0.001, n=6). siRNA-mediated knockdown of p38 has a similar effect: cells transfected with p38 siRNA had significantly lower levels of PN-induced apoptosis (14.1% 1.0) than cells transfected with PN (p=0.001, n=6). In addition, cells transfected with with p38-siRNA and treated with PN had significantly lower levels of apoptosis compared to cells transfected with control (mock)-siRNA and treated with PN (40.7% 3.8) (p=0.001, n=6). There was no difference in the rates of PN-induced apoptosis between cells pretreated with SB202190 plus PN, and those transfected with p38 siRNA plus PN (p=0.48, n=6). These data show that p38 is an essential mediator of PN-induced apoptosis. Data are noted as mean SEM.
PN decreases activating phosphorylation of AKT
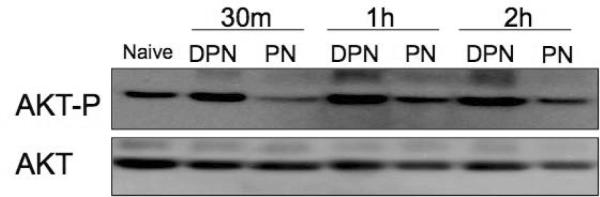
Having established the essential role of p38 in PN-induced enterocyte apoptosis, we set out to assess the role of the AKT survival pathway. IEC-6 cells were exposed to PN and allowed to recover for varying times. Untreated cells displayed relatively high levels of phospho-AKT (Fig 3A). PN, but not DPN, decreased AKT phosphorylation as early as 30 min, and the hypophosphorylated state lasted up to 2h.
Figure 3. AKT phosphorylation in IEC-6 cells treated with PN.
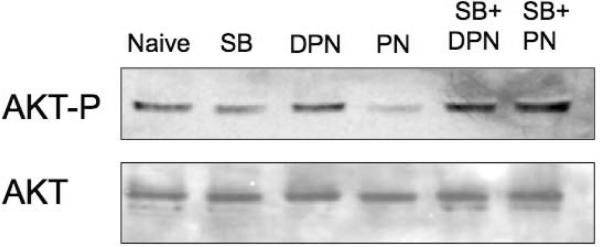
(A) Cells were treated with PN, or equivalent concentration of DPN for indicated time. Levels of phospho-AKT and total AKT were determined by Western blotting. (B) Levels of phospho-AKT and total AKT in cells untreated naive, or treated with PN or DPN for 1h, with or without pre-treatment with 10 M SB202190 (SB) as indicated. Data are representative of 3 independent experiments.
We next tested if PN-induced decrease in AKT phosphorylation depends on p38. Cells were treated with PN, with or without SB202190 pretreatment. Pharmacologic blockade of p38 abrogated the PN-induced decrease in AKT phosphorylation (Fig 3B), indicating that p38 is required for this response.
PN increases phoshoprotein phosphatase Type 2A (PP2A) activity
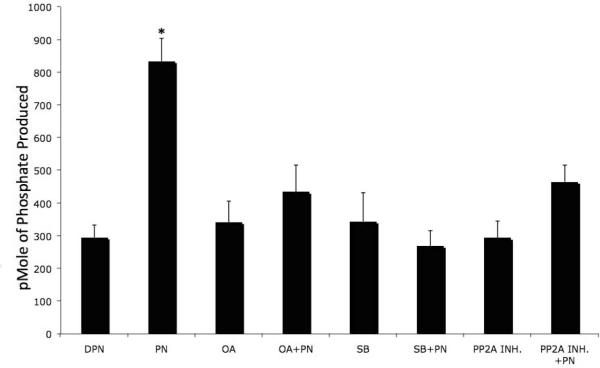
PP2A is known to regulate both PI 3-K-AKT and p38 MAPK pathways[17]. PP2A is the major phosphatase that deactivates AKT [17], and, as a negative regulator of AKT, it has been implicated in TNF-induced p38-mediated endothelial cell apoptosis [18]. We tested whether PP2A is involved in PN-induced p38-mediated apoptosis in. IEC-6 cells. Treatment with PN, but not DPN, increased PP2A activity (p<0.001, n=5) (Fig 4). Neither OA (1 M) nor I1 PP2A (100nM), the specific inhibitors of PP2A, had any effect on basal PP2A activity (Fig 4). Similarly, SB202190 (10 M) alone did not increase PP2A activity. Both OA and I1 PP2A blocked PN-induced increase in PP2A activity, as expected. PN-induced increase in PP2A activity was also abrogated by the p38 inhibitor SB202190 (Fig 4). Effects of the three inhibitors on PN-induced PP2A activation was significant (p<0.001, n=5). These data suggest that PP2A may be an important phosphatase associated with the p38 pathway.
Figure 4. PN increases PP2A activity.
IEC-6 cells were treated with PN (50 M) or equivalent concentration of DPN for 1 h. Cells were pre-treated with 10 M SB202190 (SB), 10 M okadaic acid (OA) or 100nM I1PP2A (PP2A INH) 30m prior to PN or DPN exposure. PP2A activity in cell lysates were measured. (*) PN exposure significantly elevated PP2A activity compared to all other variables (p<0.001, n=5). Data are shown as pmoles phosphate produced per mg protein in the form of mean S.E.
DISCUSSION
Sustained overproduction of NO is a hallmark of epithelial injury and gut barrier failure in NEC [19]. Much of the injury caused by NO overproduction is linked to reactive nitrogen and oxygen species. We have previously shown that PN causes enterocyte apoptosis, inhibits enterocyte proliferation, and interferes with epithelial restitution [4] [8; 9]. In this report we explored the molecular mechanisms by which PN activates apoptotic pathways. To establish a connection between the cytotoxic effects of PN and the p38 MAPK pathway, we examined PN-induced activation of MKK3/6, p38, and ATF-2. All three of these components of the p38 signaling cascade were activated by PN. Moreover, PN-induced enterocyte apoptosis was abrogated by chemical or genetic inhibition of p38, pointing to p38 activation as a key regulatory step. Our study agrees with published observations of the critical role of the p38 signaling cascade in stress-induced apoptosis in enterocytes [20; 21], but further delineates some of the intricate upstream and downstream signaling mechanisms involved.
Our results provide an important insight into the mechanisms by which activated p38 promotes apoptosis. Treatment with PN deactivates AKT, the pro-survival protein kinase that is activated by PI3-K. Importantly, pre-treatment with a p38 pharmacologic inhibitor or transfection with p38 siRNA blocks PN-induced decrease in the activating phosphorylation of AKT, which establishes the role of p38 as an inhibitor of the pro-survival signaling via AKT. Moreover, because activation of p38 increases activity of the protein phosphatase PP2A, a known negative regulator of AKT [8], it is likely that PP2A mediates inhibition of AKT by p38. Although p38-mediated activation of PP2A has been previously reported [22], our study is the first to establish its activation as a result of PN exposure in an intestinal cell line.
PP2A has been found to be the most important AKT phosphatase [17] and it has been implicated, alongside with p38, as a mediator of TNF- -induced endothelial cell apoptosis [18]. In IEC-6 enterocytes, pharmacologic inhibition of PP2A protects from TNF- -induced apoptosis [23]. Here, we demonstrate for the first time that PP2A is involved in p38 MAPK pathways in enterocytes stressed with PN. PP2A may not be the only protein serine-threonine phosphatase that inhibits the pro-survival signaling via AKT. However, it is the most abundant mammalian serine/threonine phosphatase, and it was previously linked to the apoptotic signaling [24] [25]. PP2A-mediated deactivation of AKT may be one of the mechanisms by which p38 promotes apoptosis following exposure to PN. Other mechanisms may involve down-regulation of proliferative signaling via the extracellular response kinase (ERK) [26], or p38/ATP-2-dependent induction of pro-apoptotic genes such as the growth arrest- and DNA damage-inducible protein Gadd45 [40-42]. Contribution of these mechanisms into PN-induced apoptosis in enterocytes remains to be established.
The involvement of the PI3-K cascade in PN-induced apoptosis has been described in several cell types. Transfection of endothelial cells with constitutively active AKT partially protects them from PN-induced apoptosis [45]. In the pheochromocytoma cell line PC-12, PN-induced apoptosis is associated with increased phosphorylation of AKT. However, in PC-12 cells, pharmacologic inhibition of p38 fails to prevent the PN-induced dephosphorylation of AKT, which rules out the role of p38 [10]. Thus, the pro-apoptotic p38-PP2A-AKT cascade may be a cell type-specific mechanism that operates in enterocytes, but not other cell types. p38, a member of the MAPK family, is detected at high levels in its phosphorylated (activated) form in NEC [20]. Furthermore, p38 is linked to oxidative stress-induced apoptosis in RIE-1 cells in vitro [20].
Although several studies implicate p38 as a regulator of oxidative stress-induced apoptosis in a variety of cell types, our study demonstrates the role of this MAPK as an integral component of enterocyte apoptosis secondary to nitrosative stress. Our analyses also demonstrate that PN exposure leads to deactivation of the AKT survival pathway via a p38-dependent manner. PP2A activity is also increased in a p38-dependent manner suggesting the possibility that PP2A may be one of the key phosphatases.
ACKNOWLEDGEMENTS
This investigation was supported by The Robert Wood Johnson Foundation SU401 and National Institutes of Health 1K08 GM00696-01 grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49–57. doi: 10.1053/j.sempedsurg.2004.10.025. [DOI] [PubMed] [Google Scholar]
- [2].Guner YS, Chokshi N, Petrosyan M, Upperman JS, Ford HR, Grikscheit TC. Necrotizing enterocolitis--bench to bedside: novel and emerging strategies. Semin Pediatr Surg. 2008;17:255–65. doi: 10.1053/j.sempedsurg.2008.07.004. [DOI] [PubMed] [Google Scholar]
- [3].Ford HR. Mechanism of nitric oxide-mediated intestinal barrier failure: insight into the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 2006;41:294–9. doi: 10.1016/j.jpedsurg.2005.11.003. [DOI] [PubMed] [Google Scholar]
- [4].Upperman JS, Potoka D, Grishin A, Hackam D, Zamora R, Ford HR. Mechanisms of nitric oxide-mediated intestinal barrier failure in necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:159–66. doi: 10.1053/j.sempedsurg.2005.05.004. [DOI] [PubMed] [Google Scholar]
- [5].Nathan C. Nitric oxide as a secretory product of mammalian cells. Faseb J. 1992;6:3051–64. [PubMed] [Google Scholar]
- [6].Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A. 1992;89:3030–4. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zamora R, Bryan NS, Boyle P, Wong C, Milsom AB, Jaffe R, Feelisch M, Ford HR. Nitrosative stress in an animal model of necrotizing enterocolitis. Free Radic Biol Med. 2005;39:1428–37. doi: 10.1016/j.freeradbiomed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- [8].Potoka DA, Upperman JS, Nadler EP, Wong CT, Zhou X, Zhang XR, Ford HR. NF-kappaB inhibition enhances peroxynitrite-induced enterocyte apoptosis. J Surg Res. 2002;106:7–14. doi: 10.1006/jsre.2002.6423. [DOI] [PubMed] [Google Scholar]
- [9].Potoka DA, Upperman JS, Zhang XR, Kaplan JR, Corey SJ, Grishin A, Zamora R, Ford HR. Peroxynitrite inhibits enterocyte proliferation and modulates Src kinase activity in vitro. Am J Physiol Gastrointest Liver Physiol. 2003;285:G861–9. doi: 10.1152/ajpgi.00412.2002. [DOI] [PubMed] [Google Scholar]
- [10].Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–8. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- [11].Shen YH, Zhang L, Gan Y, Wang X, Wang J, LeMaire SA, Coselli JS, Wang XL. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem. 2006;281:7727–36. doi: 10.1074/jbc.M511105200. [DOI] [PubMed] [Google Scholar]
- [12].Anderson P. Kinase cascades regulating entry into apoptosis. Microbiol Mol Biol Rev. 1997;61:33–46. doi: 10.1128/mmbr.61.1.33-46.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Upperman JS, Potoka DA, Zhang XR, Wong K, Zamora R, Ford HR. Mechanism of intestinal-derived fungal sepsis by gliotoxin, a fungal metabolite. J Pediatr Surg. 2003;38:966–70. doi: 10.1016/s0022-3468(03)00135-0. [DOI] [PubMed] [Google Scholar]
- [14].Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–5. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- [15].Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–91. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- [16].Sheikh MS, Hollander MC, Fornance AJ., Jr Role of Gadd45 in apoptosis. Biochem Pharmacol. 2000;59:43–5. doi: 10.1016/s0006-2952(99)00291-9. [DOI] [PubMed] [Google Scholar]
- [17].Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–91. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- [18].Grethe S, Porn-Ares MI. p38 MAPK regulates phosphorylation of Bad via PP2A-dependent suppression of the MEK1/2-ERK1/2 survival pathway in TNF-alpha induced endothelial apoptosis. Cell Signal. 2006;18:531–40. doi: 10.1016/j.cellsig.2005.05.023. [DOI] [PubMed] [Google Scholar]
- [19].Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 1997;32:275–82. doi: 10.1016/s0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- [20].Zhou Y, Wang Q, Evers BM, Chung DH. Signal transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis. Pediatr Res. 2005;58:1192–7. doi: 10.1203/01.pdr.0000185133.65966.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zheng SY, Fu XB, Xu JG, Zhao JY, Sun TZ, Chen W. Inhibition of p38 mitogen-activated protein kinase may decrease intestinal epithelial cell apoptosis and improve intestinal epithelial barrier function after ischemia- reperfusion injury. World J Gastroenterol. 2005;11:656–60. doi: 10.3748/wjg.v11.i5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Westermarck J, Li SP, Kallunki T, Han J, Kahari VM. p38 mitogen-activated protein kinase-dependent activation of protein phosphatases 1 and 2A inhibits MEK1 and MEK2 activity and collagenase 1 (MMP-1) gene expression. Mol Cell Biol. 2001;21:2373–83. doi: 10.1128/MCB.21.7.2373-2383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ray RM, Bhattacharya S, Johnson LR. Protein phosphatase 2A regulates apoptosis in intestinal epithelial cells. J Biol Chem. 2005;280:31091–100. doi: 10.1074/jbc.M503041200. [DOI] [PubMed] [Google Scholar]
- [24].Santoro MF, Annand RR, Robertson MM, Peng YW, Brady MJ, Mankovich JA, Hackett MC, Ghayur T, Walter G, Wong WW, Giegel DA. Regulation of protein phosphatase 2A activity by caspase-3 during apoptosis. J Biol Chem. 1998;273:13119–28. doi: 10.1074/jbc.273.21.13119. [DOI] [PubMed] [Google Scholar]
- [25].Garcia A, Cayla X, Guergnon J, Dessauge F, Hospital V, Rebollo MP, Fleischer A, Rebollo A. Serine/threonine protein phosphatases PP1 and PP2A are key players in apoptosis. Biochimie. 2003;85:721–6. doi: 10.1016/j.biochi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- [26].Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. Faseb J. 2008;22:954–65. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]