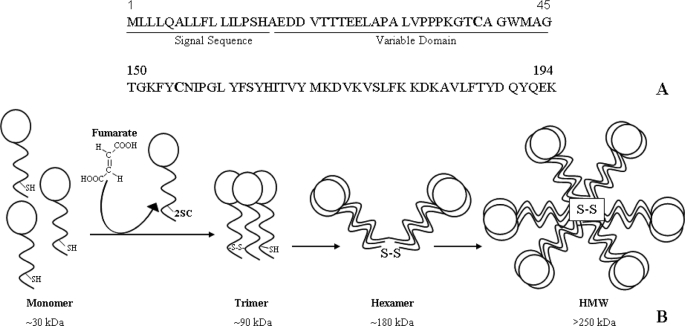
FIGURE 1.
Structure of adiponectin. Two cysteines are highly conserved in adiponectin monomer: one in the hypervariable region adjacent to the N terminus (Cys-39) and the other in the C-terminal globular head domain (Cys-155) (A). Adiponectin monomers associate into trimers through disulfide bonding, and trimers associate through disulfide bonds to form LMW and HMW multimers, which are secreted from the adipocyte. Succination of Cys-39 blocks incorporation of adiponectin monomer into trimer and higher molecular weight secreted forms of the protein (B).