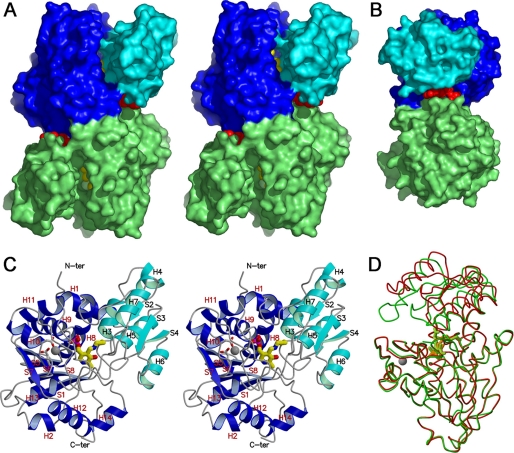
FIGURE 2.
Dimeric and monomeric structures of spSR. A, stereo view of the spSRw complex with AMP-PCP in the open form along the two-fold axis. The small and large domains of one subunit are shown in light and deep blue, respectively, and the other subunit is shown in green. A large deep groove is formed at the domain interface. The cofactor PLP shown by a space-filling model (yellow) is bound to the bottom of the groove. The groove extends to the subunit interface. AMP-PCP (red) is bound to the groove located at the boundary between the domain interface and the subunit interface on the right or left side of the molecule. N-ter, N terminus; C-ter, C terminus. B, side view of the molecule perpendicular to the two-fold axis. The image structure is turned 90° counterclockwise around the vertical axis relative to the image in Fig. 1A. The groove embracing AMP-PCP is further extended to the subunit interface formed at the back of the molecule. Binding of AMP-PCP induces the rotation of both subunits to widen the back groove. C, stereo view of the subunit in the open form with the secondary structure assignments. The small and the large domains are shown in light and deep blue, respectively. The cofactor PLP-Lys-57 Schiff base located at the bottom of the groove is drawn as the stick model. Besides the cofactor, a metal ion (gray circle) is bound to the large domain to stabilize the active site folding. The metal ion is coordinated by the side-chain carboxylates of Glu-208 and Asp-214. D, large (lower half) domain fitting between spSRw in the open form (red) and spSRm in the closed form (green). The large domain superimposition reveals the rotation of the small domain toward the large domain to close the active site.