Abstract
Matrix metalloproteinase-13 (MMP-13, or collagenase 3) has been shown to degrade intact collagen and to participate in situations where rapid and effective remodeling of collagenous ECM is required. Mechanical strain induction of MMP-13 is an example of how osteoblasts respond to high mechanical forces and participate in the bone-remodeling mechanism. Using MC3T3-E1 osteoblast-like cells, we dissected the signaling molecules involved in MMP-13 induction by mechanical strain. Reverse transcription-PCR and zymogram analysis showed that platelet-derived growth factor receptor (PDGFR) inhibitor, AG1296, inhibited the mechanical strain-induced MMP-13 gene and activity. However, the induction was not affected by anti-PDGF-AA serum. Immunoblot analysis revealed time-dependent phosphorylation of PDGFR-α up to 2.7-fold increases within 3 min under strain. Transfection with shPDGFR-α (at 4 and 8 μg/ml) abolished PDGFR-α and reduced MMP-13 expression. Moreover, time-dependent recruitments of phosphoinositide 3-kinase (PI3K) by PDGFR-α were detected by immunoprecipitation with anti-PDGFR-α serum followed by immunoblot with anti-PI3K serum. AG1296 inhibited PDGFR-α/PI3K aggregation and Akt phosphorylation. Interestingly, protein kinase C-δ (PKC-δ) inhibitor, rottlerin, inhibited not only PDGFR-α/PI3K aggregation but PDGFR-α phosphorylation. The sequential activations were further confirmed by mutants ΔPKC-δ, ΔAkt, and ΔERK1. Consistently, the primary mouse osteoblast cells used the same identified signaling molecules to express MMP-13 under mechanical strain. These results demonstrate that, in osteoblast-like cells, the MMP-13 induction by mechanical strain requires the transactivation of PDGFR-α by PKC-δ and the cross-talk between PDGFR-α/PI3K/Akt and MEK/ERK pathways.
Mechanical strain to bone is considered to be important for the maintenance of bone integrity and architecture. The process of bone (re)modeling under mechanical loading may repair fatigue damage and improve bone strength (1–3). Such (re)modeling requires bone resorption and deposition by the concerted efforts of osteoblasts and osteoclasts. Several studies have demonstrated that, in the absence of the systemic and local factors, mechanical loading on osteoblasts in vitro is able to increase prostaglandin release (4), stimulate cell division (5), alter collagen synthesis (6), and promote collagenase activity (7). Other induced proteins such as insulin-like growth factors I and II, transforming growth factor-β, osteocalcin, osteopontin, nitric-oxide synthase, and cyclooxygease-2 have also been reported (8).
Previously, we reported that mechanical strain induces collagenase 3 (MMP-13)2 expression by MC3T3-E1 osteoblast-like cells (9). The MMP-13 mRNA induction is transient, stable, and does not require de novo protein synthesis, suggesting that an immediate action be taken by strained osteoblasts to participate in the resorption phase of matrix (re)modeling. MMP-13 is a neutral proteinase capable of degrading native fibrillar collagens in the extracellular space (10, 11). It may be involved in situations where rapid and effective remodeling of collagenous extracellular matrix is required. Hence, MMP-13 can be detected in primary fetal ossification during bone morphogenesis, and in remodeling of the mature skeletal tissue (12, 13).
Mechanical strain induction of MMP-13 may be mediated through a process of mechanotransduction, converting physical forces into biochemical signals and integrating these signals into cellular responses. In our stretch chamber system, we showed that the mechanotransduction utilizes the MEK/ERK signaling pathway to implement MMP-13 expression (9). However, the transduction mechanism involved remains unclear and awaits further investigation. Three lines of studies have prompted us to investigate the receptor of platelet-derived growth factor receptor (PDGFR) as a potential mechanoreceptor in the MMP-13 induction. PDGF-BB induces MMP-13 expression in osteoblasts (14, 15), whereas in vascular smooth muscle cells the mechanical strain increases PDGF-B and PDGFR-β expression (16) and activates PDGFR-α (17).
The PDGFRs, including PDGFR-α and -β, are membrane glycoproteins of ∼170 and 180 kDa, respectively. Their structures are similar to those of the colony-stimulating factor-1 receptor and the stem cell factor receptor. The extracellular parts of PDGFR consist of five immunoglobulin-like domains, among which three outer-most domains are for ligand binding, and domain 4, for direct receptor-receptor interactions. The intracellular parts contain a tyrosine kinase domain, with characteristic inserted sequences without homology to kinases (18). The PDGFR-α binds all combinations of PDGF-A/-B forms, whereas PDGFR-β binds only PDGF-BB. The binding of the ligand induces dimerization of the PDGFR, leading to the activation via autophosphorylation of tyrosine residues in the PDGFR kinase domain. Inside the kinase domains, autophosphorylation increases the kinase activity, whereas, outside of it, autophosphorylation creates docking sites for the recruitment of cytoplasmic molecules containing SH domains as in enzyme, PI3K, or in adaptor protein, Grb2.
To dissect the sequential signaling involved in the MMP-13 induction by mechanical strain, we applied mechanical stretching to MC3T3-E1 osteoblast-like cells grown on a collagen-coated flexible membrane in the presence of inhibitors and dominant mutants of interest. We found that in osteoblast-like cells, the mechanical strain induced MMP-13 expression requires transactivation of PDGFR-α by PKC-δ.
EXPERIMENTAL PROCEDURES
Materials
Murine MC3T3-E1 cell line was used as a homogeneous source of non-transformed osteoblast-like cells. Primary osteoblast cells were obtained from calvaria of neonatal mice (ICR-CD1) through standard protocol of collagenase digestion (19).
Fetal bovine serum, TRIzol, and minimal essential medium-α (α-MEM) were purchased from Invitrogen. The anti-MMP-13 monoclonal antibody was purchased from NeoMarkers (Fremont, CA); anti-PDGF-AA serum, from R&D (Minneapolis, MN), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antiserum, from Biogenesis (Boumemouth, UK). The antibodies against PDGFR-α, PDGFR-β, and PI3K were from Santa Cruz Biotechnology (Santa Cruz, CA), whereas antibodies against phospho-PDGFR-α, phospho-PDGRF-β, and phospho-p42/p44 MAPK were from Cell Signaling (Beverly, MA). The reagents AG1296, AG1478, genistein, herbimycin A, rottlerin, GF109203X, Gö6976, and LY294002 were from Biomol (Plymouth Meeting, PA). Bicinchoninic acid (BCA) protein assay kit was from Pierce. Enhanced chemiluminescence (ECL) immunoblotting detection system and Hyperfilms were from Amersham Biosciences. Type I collagen, enzymes, and other chemicals were from Sigma. Dominant negative mutants of ERK1 (ERK1 K52R), Akt, and PKC-δ were generously provided by Drs. M. H. Cobb (Dept. of Pharmacology, University of Texas Southwestern Medical Center, Houston, TX), R. D. Ye (Dept. of Pharmacology, University of Chicago, Chicago, IL), and P. Parker (Cancer Research Center, London, UK), respectively.
In Vitro Equibiaxial Stretch Device
The equibiaxial stretch chamber (9), modified from the work of Lee et al. (20) was used to deliver uniform, isotropic, and static tensile strain to osteoblast-like cells in the absence of shear as previously described. The chambers were H2O2 gas-sterilized before use. Similar to the study of gene expression by applying a cyclic 8% stretch at a frequency of 0.5 Hz to human chondrosarcoma (21), in the present experiments, we used 8% stretch for the optimal expression of MAPKs as a reference. Nevertheless, to exclude the release of intracellular MMP-13 as a result of cell injury, media conditioned by control or tested cells was assayed for lactate dehydrogenase (22, 23). There was no significant difference in release of lactate dehydrogenase in the medium, and no slippage of the strained cells from the collagen-coated membrane with a prolonged period of time (9).
The MC3T3-E1 osteoblast-like cells were grown in flasks to sub-confluence in α-MEM containing 10% fetal bovine serum before plating to stretch chamber. The elastic sheets of the chamber were coated with a solution of 0.01% type I collagen overnight to promote cell attachment. Then, the MC3T3-E1 cells were transferred and plated at a density of 1 × 105 cells/cm2 to the collagen-coated sheet and grown to confluence. After conditioning in serum-free α-MEM medium overnight, quiescent adherent cells were stretched under testing condition. For experimental purposes, the selective inhibitors (all at 10 μm concentration) were added 1 h before testing. Control cells were treated in an identical fashion as test cells, yet without being stretched. Test and control experiments were carried out simultaneously with the same pool of cells in each experiment to match temperature, CO2 content, and pH of the medium for the test and control cells.
Zymogram Analysis
Briefly, aliquots of the control and test media were electrophoresed on a 10% SDS-polyacrylamide gel containing 1.25% gelatin. Afterward, the gel was washed with 2.5% Triton X-100 to remove SDS, rinsed with 50 mm Tris-HCl, pH 7.5, and then incubated overnight at room temperature with the developing buffer (50 mm Tris-HCl, pH 7.5, 5 mm CaCl2, 1 μm ZnCl2, 0.02% thimerosal, 1% Triton X-100). The zymographic activities were revealed by staining with 1% Coomassie Blue and later, destaining of the gel and were quantified by laser densitometry of the corresponding bands in the linear response of the gelatin zymogram.
RNA Isolation, Reverse Transcription, and PCR
The adherent cells were harvested after being stretched for the time indicated. Total RNA was isolated using TRIzol reagent according to the manufacturer's instructions and quantified by optical density. 1 μg of total RNA was added to a reverse transcriptase (RT) reaction in RT buffer containing 20 mm Tris-HCl (pH 8.4), 50 mm KCl, and 2.5 mm MgCl2, 10 mm dNTPs, 0.1 m dithiothreitol, 0.5 mg of oligo(dT) primer, 200 units of SuperScript II RT, and RNase H. 5 μl of cDNA from the RT was added directly to a 50-μl PCR containing 20 mm Tris-HCl (pH 8.4), 50 mm KCl, 25 mm MgCl2, 10 mm dNTP, 2.5 units of TaqDNA polymerase. The amplification conditions for MMP-13 were as follows: 94 °C/1 min, 62 °C/1 min, and 72 °C/2 min, which was amplified for 30 cycles. Oligonucleotide primers were designed to span at least one intron to detect any contaminating genomic DNA carried over from the RNA isolation step. The β-actin primer sequences have been described previously (Ambion), and MMP-13 primer sequences were derived from the mouse MMP-13 sequence (24) as follows: MMP-13, sense primer 5′-GGT CCC AAA CGA ACT TAA CTT ACA-3′ and antisense primer 5′-CCT TGA ACG TCA TCA TCA GGA AGC-3′, a total of 445 bp. Conditions were established so that PCR was stopped in the linear range, and the reaction products could be accurately quantified and compared. PCR products were electrophoresed on 1.5% agarose gels. Ethidium bromide staining of the bands corresponding to MMP-13 was photographed and digitized. Density analysis was performed using the UN-SCAN-IT gel program (Silk Scientific, Inc. Orem, UT). The levels of MMP-13 mRNA were normalized to those of β-actin RNA to correct for differences in loading and/or transferring.
Preparation of Cell Extracts and Immunoblot Analysis of Signaling Molecules
Unless mentioned otherwise, protein concentrations were determined (25) with bovine serum albumin as the standard. MC3T3-E1s in control or in test (by 8% stretch) groups were incubated for various times before subjected to cell lysis as described previously (9). At the termination of mechanical stimulation, cells were rapidly washed with ice-cold phosphate-buffered saline twice, and lysed on ice in 0.2 ml of lysis buffer (containing 25 mm Tris-HCl, pH 7.4, 25 mm NaCl, 25 mm NaF, 25 mm Na4P2O7, 1 mm Na2VO4, 2.5 mm EGTA, 2.5 mm EDTA, and 0.05% Triton X-100, 0.5% Nonidet P-40, 0.5% SDS, 0.5% deoxycholate, and protease inhibitors such as 5 μg/ml leupeptin, 5 μg/ml aprotinin, and 1 mm phenylmethylsulfonyl fluoride). The lysates were centrifuged at 45,000 × g for 1 h at 4 °C to yield the cell extract. Equal amounts of samples were electrophoresed on a 10% polyacrylamide gel and were then blotted to nitrocellulose membrane. Subsequently, the membrane was incubated at room temperature with 5% bovine serum albumin in TTBS (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.05% Tween 20) for 1 h. The total protein profiles and the phosphorylated forms of the kinases were identified by immunoblot analysis with anti-serum raised against the signaling molecules or their phosphorylated forms. Briefly, membranes were incubated with a 1:1000 diluted solution of specific anti-PDGF-α, anti-phospho-PDGFR-α, anti-phospho-Akt, anti-phospho-p42/p44 MAPK, anti-PI3K, or anti-GAPDH antibodies, and then with the second antibody (anti-rabbit horseradish peroxidase antibody in 1% bovine serum albumin/TTBS; 1:1500 dilution). Immunoreactive bands were visualized by using an enhanced chemiluminescent (ECL) system.
Coimmunoprecipitation Assay
Cell lysates containing 1 mg of protein were incubated with 2 μg of anti-PDGFR-α antibody at 4 °C for 1 h, and then 10 μl of 50% protein A-agarose beads was added and mixed for 16 h at 4 °C. The immunoprecipitates by anti-PDGFR-α serum were collected and washed three times with lysis buffer without Triton X-100, dissolved in 5× Laemmli buffer, and then subjected to electrophoresis on 10% SDS-PAGE. Immunoblot analysis was performed using anti-PI3K and anti-PDGFR-α serum.
Plasmid Construction
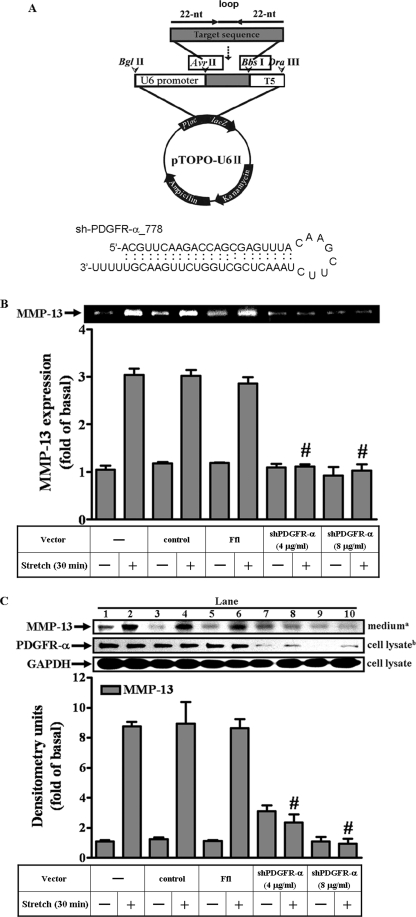
The vector pTOPO-U6 II was constructed by inserting the short hairpin RNA expression cassette containing the mouse U6 promoter and the termination signal of RNA polymerase III into the pCRII-TOPO vector (Invitrogen), the procedure of which was similar to that of the pTOPO-U6 construction (26). Minor modifications were made to build the AvrII and BbsI sites outside of the inserted, designed 22-oligomer short hairpin RNA sequences (Fig. 3A). For the plasmid PDGFR-α_778, and PDGFR-β_2572, the complementary oligonucleotides PDGFR-α_778 sense (5′-CTAGACGTTCAAGACCAGCGAGTTTACAAGCTTCTAAACTCGCTGGTCTTGAACGT-3) and PDGFR-α_778 antisense (5′-AAAAACGTTCAAGACCAGCGAGTTTAGAAGCTTGTAAACTCGCTGGTCTTGAACGT-3′), and PDGFR-β_2572 sense (5′-CTAGGGCATGGACTTCTTAGCCTCTACAAGCTTCTAGAGGCTAAGAAGTCCATGCC-3′) and PDGFR-β_2572 antisense (5′-AAAAGGCATGGACTTCTTAGCCTCTAGAAGCTTGTAGAGGCTAAGAAGTCCATGCC-3′) were annealed, respectively, as described by Tseng et al. (26). For small interfering RNA of firefly luciferase, the complementary oligonucleotides FflS and FflAS were constructed and reported previously (27).
FIGURE 3.
MMP-13 expression required the presence of PDGFR-α. Schematic representation of PDGFR-α short hairpin RNA expression plasmid and the putative structure of the dsRNA formed by sh-PDGFR-α_778 were shown (A). The plasmids for vector control (pTOPO-U6II), small interfering RNA for firefly luciferase (Ffl) and shPDGFR-α were transiently transfected into MC3T3-E1 osteoblast-like cells. Cells were incubated for 24 h, and then serum-free conditioned overnight before testing for 30 min. The culture medium and cell lysates were collected for the immunoblot analysis of MMP-13 and PDGFR-α, respectively (C). The MMP-13 activities were revealed by zymogram (B). The vector control or Ffl alone did not affect MMP-13 and PDGFR-α (B and C). However, two doses of the shPDGFR-α at 4 and 8 μg/ml (B and C) blocked PDGFR-α and attenuated MMP-13 and its activities. #, p < 0.01, as compared with the strained cells. This was a result of four sets of independent experiments.
Plasmids and Transfection
The plasmids encoding ΔERK1 K52R, ΔMEK K97R, and ΔPKC-δ (dominant negative mutants of ERK1 and MEK1/2) were prepared by using Qiagen plasmid DNA preparation kits. For transfection, the amount of plasmid (1 μg) was kept constant for each experiment. The DNA PLUS-Lipofectamine reagent complex was prepared according to the instructions of the manufacturer (Invitrogen). The adherent MC3T3-E1 cells grown to 70% confluence were washed once with phosphate-buffered saline and then with serum-free α-MEM medium and then transfected and incubated with plasmid in serum-free α-MEM (0.8 ml) and DNA PLUS-Lipofectamine reagent (0.2 ml) at 37 °C for 5 h and later with α-MEM (1 ml) containing 10% fetal bovine serum overnight. After 24 h of transfection, cells were washed twice with phosphate-buffered saline and maintained in α-MEM containing 10% fetal bovine serum for an additional 24 h. Before applying an 8% stretch, cells were washed once with phosphate-buffered saline and incubated with serum-free α-MEM for 24 h.
Statistic Analysis
Data were presented as mean ± S.D. Statistical comparisons of control group with treated groups were carried out using the paired sample t test with p values corrected by the Bonferroni method. Comparisons among three or more groups were made by one-way analysis of variance followed by Dunnett's post hoc analysis. An effect was considered significant when p < 0.05.
RESULTS
Activation of the PDGFR-α Leads to the MMP-13 Induction by Mechanical Strain
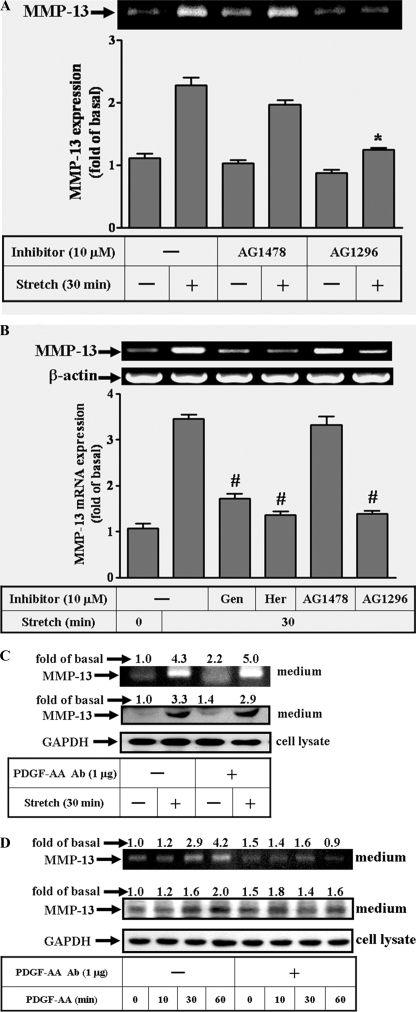
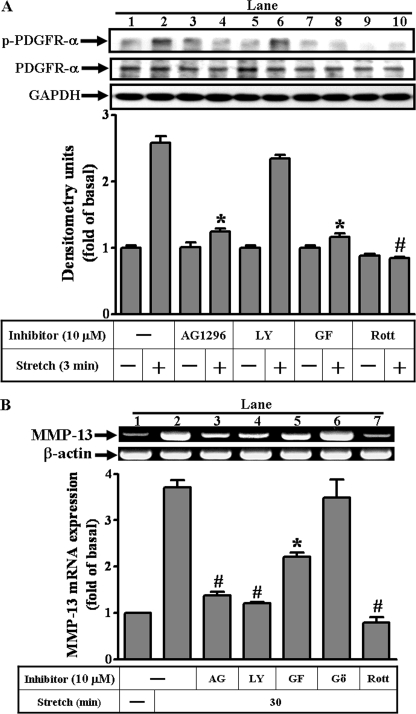
In this study, we investigated whether the signaling molecules at the level of plasma membrane-associated receptor tyrosine kinases may be involved in MMP-13 expression by mechanical strain. The inhibitors used as diagnostic tools included genistein and herbimycin A for blocking the protein-tyrosine kinases in general, and specifically, AG1296 and AG1478 for blocking the receptor tyrosine kinases such as PDGFR and EGFR, respectively. The zymogram revealed that the mechanical strain-induced MMP-13 activities were partly blocked by pretreatment with AG1296 (Fig. 1A). However, AG1478 failed to inhibit the activities. Moreover, the RT-PCR analysis reflected the above finding of the AG1296 by matching the reduction of MMP-13 gene with that of activity under mechanical insults (Fig. 1B). Taken together, these data suggested that the PDGFR participates in the mechanical strain-induced MMP-13 expression.
FIGURE 1.
Effects of the inhibitors on the MMP-13 induction by mechanical strain in the osteoblast-like cells. The adherent cells were grown on a collagen-coated flexible sheet, conditioned overnight, pre-treated with inhibitors or anti-PDGF-AA serum (1 μg) 1 h before testing and then stretched at 8% for 30 min. The inhibitors were genistein (10 μm), herbimycin A (10 μm), AG1478 (10 μm, for blocking EGFR), and AG1296 (10 μm, for blocking PDGFR). The MMP-13 activities were revealed indirectly by zymogram (A), whereas MMP-13 proteins were analyzed by immunoblot (C and D). Inhibitor AG1478 or AG1296 alone did not alter the basal MMP-13 activities. The agent PDGF-AA (10 μm) and its counterpart anti-PDGF-AA serum were included for detecting MMP-13 expression (D). Total RNAs from control and strained cells were analyzed by RT-PCR using mRNAs of the MMP-13 and actin as templates (B). The amplified bands were revealed by ethidium bromide staining and quantified by the UN-SCAN-IT gel program as described under “Experimental Procedures.” Representative mRNA levels of MMP-13 (B) and β-actin (as an internal control) are shown. In general, the minimum activity from the control group during the time course experiments is designated as 1. Significant difference was leveled as *, p < 0.05 or #, p < 0.01 in comparison with the unstrained cells. This was a result of three sets of independent experiments.
Because PDGF-A mRNA expression was reported to be induced by mechanical strain within 2 h in MC3T3-E1s (28), one may question whether MMP-13 expression was a secondary effect as a result of PDGF-AA production induced by mechanical insult to cells. Here, pretreatment with anti-PDGF-AA serum did not interfere with MMP-13 activities, and protein expression induced by mechanical strain (Fig. 1C). The accountability of the anti-PDGF-AA serum to neutralize PDGF-AA was assured by the following experiment. MC3T3-E1s were pretreated with PDGF-AA (10 ng/ml) to induce MMP-13 expression in the presence or absence of anti-PDGF-AA serum (Fig. 1D). The results showed that MMP-13 expressions increased by prolonged incubation with PDGF alone. However, the increased amount of MMP-13 by PDGF-AA returned to basal level in the presence of anti-PDGF-AA serum. Taken together, these data suggested that the mechanical induction of MMP-13 be independent of PDGF-AA effect.
Receptor Phosphorylation under Mechanical Strain Condition
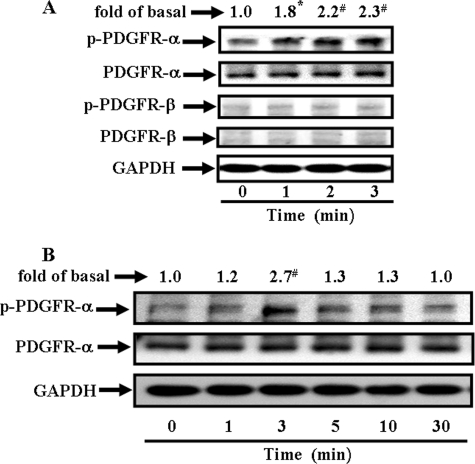
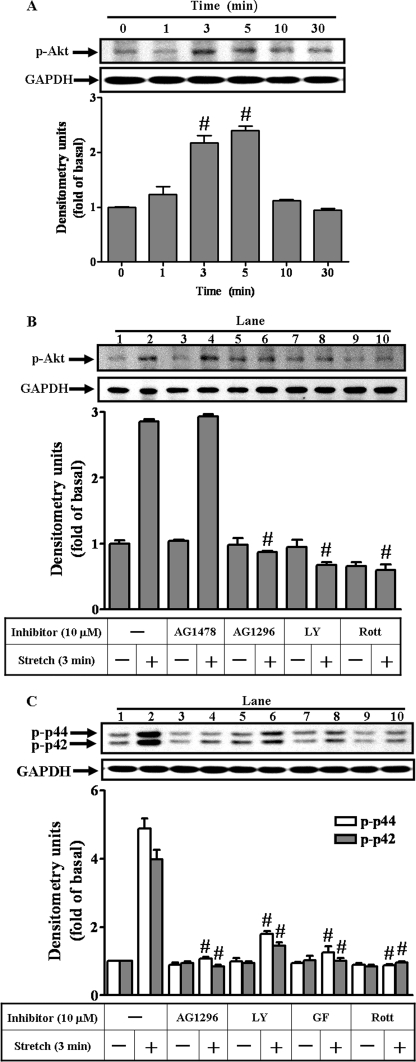
The PDGFR consists of PDGFR-α and PDGFR-β forms. We examined which of the PDGFRs might be involved in the mechanical strain-induced MMP-13 expression by immunoblot analysis with anti-PDGFR-α or with anti-PDGFR-β serum to determine the amount of the proteins present, then with anti-phospho-PDGFR-α and -β serum to detect the state of activation, or lastly with anti-GAPDH serum as an internal control (Fig. 2). We noted that only trace amounts of the PDGFR-β were present within cells. Overwhelmingly, the PDGFR-α form appeared to be dominant in quantity over PDGFR-β form (Fig. 2A, rows 2 and 4). When challenged by mechanical strain, the PDGFR-α form became phosphorylated and activated. The receptor activation of the phosphorylated PDGFR-α was a fast process. It peaked at 3 min with 2.7-fold increases and then declined gradually at 30 min (Fig. 2B). Within such a short time, obviously, the total amounts of both PDGFR-α and GAPDH did not change. On the other hand, neither PDGFR-β nor EGFR (data not shown) appeared to be activated throughout the course of challenge. Thus, these results indicated that, under a static stretch condition, the PDGFR-α phosphorylation occurred in a time-dependent manner.
FIGURE 2.
Time-dependent PDGF receptor (PDGFR) autophosphorylation under mechanical strain. The adherent cells were conditioned overnight, and then stretched at 8% for the time indicated. Immunoblot analyses were performed using antibody reactive with PDGFR-α and -β, phosphorylated PDGFR-α (p-PDGFR-α) and -β, or GAPDH (as a control). The phosphorylated profiles of the PDGFR-α and -β were aligned (A, a representative of three independent experiments) and the time-dependent receptor phosphorylations (p-PDGFR-α) were traced up to 30 min and analyzed (B). Bands were visualized by an ECL method and quantified by a densitometer. Data were summarized, plotted, and normalized independently for comparison as -fold of increases for the p-PDGFR-α (A and B) or p-PDGFR-β (A). *, p < 0.05; #, p < 0.01, as compared with the unstrained cells. This was a result of three sets of independent experiments.
PDGFR-α Participates in Mechanical Strain-induced MMP-13 Expression
To confirm the participation of the PDGFR-α in the MMP-13 induction by mechanical strain, we designed a short hairpin RNA of PDGFR-α (shPDGFR-α) (Fig. 3A) to knock down the PDGFR-α in vivo and then traced the consequences after mechanical insults. Cells were transfected with shPDGFR-α, vector alone (pTOPO-U6II), or small interfering RNA targeting firefly luciferase (Ffl) (27), incubated for additional 24 h, and afterward, serum-free conditioned overnight before testing for 30 min.
The MMP-13 activities were enhanced by mechanical strain in control cells and in cells transfected with either vector or Ffl. Such enhancements were diminished in cells transfected with the shPDGFR-α at doses of 4 and 8 μg/ml (Fig. 3, B and C). Immunoblot analysis with anti-PDGFR-α or anti-MMP-13 serum revealed that the shPDGFR-α knocked down the PDGFR-α protein expression and correspondingly, attenuated the mechanical strain-induced MMP-13 expression (Fig. 3C, lanes 7–10). Contrarily, vector or Ffl alone did not alter the amount of PDGFR-α expression (Fig. 3C, lanes 3–6). To sum up, the results confirmed that PDGFR-α, at the level of plasma membrane-associated receptor tyrosine kinase, participated in the mechanical strain induced MMP-13 expression. In line with this, the role of the PDGFR-α was further identified by the subsequent investigations in signaling relay leading to MMP-13 expression.
The Downstream Effectors of PDGFR-α: PI3K-Akt and ERK
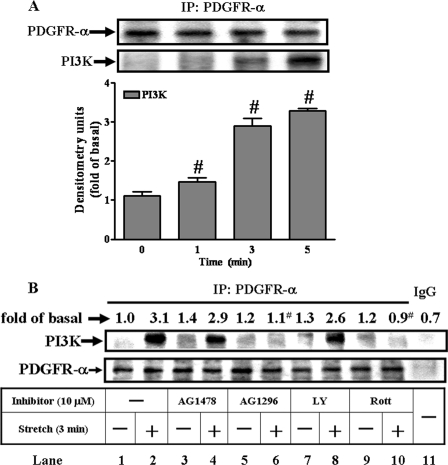
Upon activation by stimuli, the PDGFR-α recruits the cytoplasmic molecule such as PI3K that contains conserved SH domain. The PI3K, an enzyme, was identified in our stretch system via immunoprecipitation from tested MC3T3-E1s with anti-PDGFR-α serum followed by immunoblot analysis with anti-PI3K serum. Even though the amounts of PDGFR-α remained constant with or without strain, the recruitment of PI3K by PDGFR-α started within 1 min of testing and reached 3-fold increases or higher from 3 to 5 min (Fig. 4A). Such recruitments were blocked by pretreatment with AG1296 (Fig. 4B, lane 6), indicating an association of PI3K with PDGFR-α. Pretreatment with AG1478, LY294002, or IgG (Fig. 4B, lane 11) did not affect the association. The blockade by rottlerin (Fig. 4B, lanes 9–10) is separately described in the next section.
FIGURE 4.
Recruitment of PI3K by PDGFR-α. To study the time course-dependent recruitment of the PI3K by PDGFR-α, we applied the mechanical strain to the cells for the time indicated (A). Afterward, cell lysates were subjected to immunoprecipitation with anti-PDGFR-α antibody followed by immunoblot with anti-PI3K antibody as described under “Experimental Procedures.” Even though equal amounts of PDGFR-α were present in each sample (A, upper panel), the amount of PI3K increased with time, reaching maximum at 5 min. (A, lower panel), indicating the time-dependent recruitment of the PI3K by PDGFR-α. The recruitment was examined in the presence of inhibitors: AG1478, AG1296, LY294002, and rottlerin (B). The amounts of the PDGFR-α remained constant (B, upper panel), whereas the amounts of PI3K reduced significantly by AG 1296 and rottlerin (B, lower panel). #, p < 0.01, as compared with the control. This was a result of four sets of independent experiments.
To confirm the finding that the PDGFR-α/PI3K pathway was involved in MMP-13 induction, sequential activation of the PDGFR-α and PI3K complex was analyzed by immunoblot with anti-PDGFR-α and anti-phospho-PDGFR-α serum (Fig. 5A). In this experiment, pretreatment with AG1296 inhibited the mechanical strain-stimulated phosphorylation of PDGFR-α, whereas LY294002 did not (Fig. 5A, lane 6), confirming that PI3K was downstream of PDGFR-α. On the other hand, both inhibitors blocked the MMP-13 gene expression induced by mechanical strain (Fig. 5B). The effects of PKC inhibitors, including GF109203X, Gö6976, and rottlerin, on PDGFR-α phosphorylation and MMP-13 gene expression (Fig. 5, A and B) are described below. To sum up, we concluded that mechanical strain-induced MMP-13 expression was mediated via PDGFR-α/PI3K signaling.
FIGURE 5.
Involvement of the signaling molecules up- or down-stream of PDGFR-α in MMP-13 induction by mechanical strain. The adherent MC3T3-E1 cells were prepared for testing at 8% stretch for 3 or 30 min to examine PDGFR-α phosphorylation and MMP-13 gene expression. Immunoblot analysis was performed using anti-p-PDGFR-α, anti-PDGFR-α, or anti-GAPDH (as a control) antibody (A). Total RNAs from control and strained cells were amplified by RT-PCR using mRNAs of the MMP-13, and β-actin as templates. Representative mRNA levels of MMP-13 and β-actin genes (as an internal control) were shown (B). The inhibitors included in the assay were AG1296 (for PDGFR-α), LY294002 (for PI3K), GF109203X (for total PKC), Gö6976 (for calcium-dependent PKC), and rottlerin (for selective calcium-independent PKC-δ). Data are summarized and expressed as mean ± S.E. of at least three independent experiments (bar graph). *, p < 0.05; #, p < 0.01, as compared with the stretch alone.
To trace signaling relay downstream of PI3K, the PDGFR/PI3K/Akt (29) and p42/p44 MAPK pathways were studied. The phosphorylation and activation of Akt transiently occurred within 1 min and peaked within 5 min after strain application (Fig. 6A), corresponding to the optimal time frame of the PDGFR-α/PI3K recruitment. The mechanical strain-stimulated phosphorylations of Akt or p42/p44 MAPK were blocked by pretreatment with AG1296 or LY294002 (Fig. 6, B and C), suggesting that PDGFR-α/PI3K is upstream of the Akt and p42/p44 MAPK in this signaling transduction pathway.
FIGURE 6.
The Akt and p42/p44 activations by mechanical strain. The time-dependent phosphorylation of Akt was traced (A). Cells were treated under the same condition in the presence of inhibitors added 1 h before receiving 3-min strain. The Akt and p42/p44 phosphorylation were investigated (B and C) by immunoblot analysis with anti-phospho-Akt, anti-phospho-p42/p44 MAPK, or anti-GAPDH (as a control) antibody. Inhibitors (at 10 μm concentration) were the AG1478, AG1296, LY294002 (LY), GF109203X (GF), and rottlerin (Rott). The phosphorylated profiles of the Akt and p42/p44 MAPK were the representatives of four independent experiments. #, p < 0.01, as compared with the basal level (A) and stretch (B and C) alone.
The Upstream Modulator of PDGFR-α: PKC-δ
To investigate whether and which isoform of the PKCs may participate in the PDGFR-α/PI3K pathway, the PKC inhibitors, including the broad spectrum GF109203X, the calcium-dependent Gö6976, and a selective calcium-independent rottlerin (for PKC-δ), were used for these purposes. Mechanical strain-induced MMP-13 gene expression was attenuated by pretreatment with GF109203X and rottlerin (Fig. 5B, lanes 5 and 7), but not by Gö6976 (Fig. 5B, lane 6), suggesting the involvement of the calcium-independent PKC-δ (a novel PKC) in MMP-13 induction.
Mechanical strain-stimulated phosphorylation of Akt (Fig. 6B, lane 10) and p42/p44 MAPK (Fig. 6C, lane 10) was attenuated by pretreatment with rottlerin. Moreover, the interaction between PDGFR-α and PI3K was revealed by the results of the rottlerin inhibition on the PDGFR-α phosphorylation (Fig. 5A, lane 10) and on the PDGFR-α/PI3K association (Fig. 4B, lane 10) by mechanical strain. Thus, these results suggested a PKC-δ-dependent PDGFR-α transactivation mechanism in the mechanical strain-induced MMP-13 expression.
Delineation of the Signaling Cascade Leading to Mechanical Strain-induced MMP-13 Expression by Transfection with Dominant Negative Mutants of PKC-δ, Akt, and ERK1
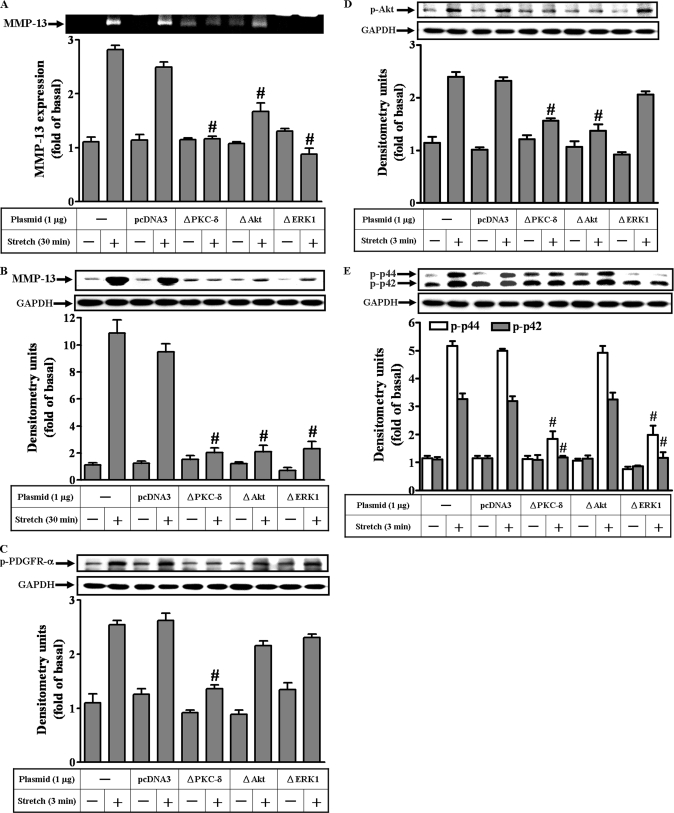
To ensure the involvement of PKC-δ, Akt, and p42/p44 MAPK in mechanical strain-induced MMP-13 expression, dominant negative mutants encoded these signaling components were used. Mutants ΔPKC-δ, ΔAkt, and ΔERK1 inhibited the expression of the MMP-13 activities and protein in strained MC3T3-E1 osteoblast-like cells (Fig. 7, A and B), in agreement with the findings of using selective pharmacological inhibitors. Moreover, ΔPKC-δ inhibited the phosphorylation of PDGFR-α, Akt, and p42/p44 MAPK (Fig. 7, C–E); whereas neither ΔAkt nor ΔERK1 succeeded in blocking PDGFR-α phosphorylation (Fig. 7C), affirming the leading role of the PKC-δ in the PDGFR-α/PI3K/Akt pathway.
FIGURE 7.
The sequential activation of the signaling molecules in MMP-13 induction by mechanical strain. Dominant negative mutants of the signaling molecules such as ΔPKC-δ, ΔAkt, and ΔERK1 were included to determine the sequential activation. First, the effects of the specific mutants on the strain-induced MMP-13 activities (A) and expressions (B) were examined. The activities and the proteins of the MMP-13 were significantly reduced by the mutants. Next, the effects of the mutants on the phosphorylations of the signaling molecules, including PDGFR-α (C), Akt (D), and p42/p44 MAPK (E) were examined. Among the mutants tested, only ΔPKC-δ inhibited the PDGFR-α phosphorylation (C). The Akt phosphorylations were inhibited by ΔPKC-δ and ΔAkt (D). The phosphorylations of the p42/p44 MAPK were blocked by ΔPKC-δ and ΔERK1. However, ΔAkt did not interfere with p42/p44 MAPK phosphorylation (E). #, p < 0.01, as compared with the control alone. This was a result of three sets of independent experiments.
Interestingly, ΔERK1 did not inhibit Akt phosphorylation (Fig. 7D). Similarly, ΔAkt did not inhibit the p42/p44 MAPK phosphorylation (Fig. 7E). Hence, no interaction occurred between p42/p44 MAPK and Akt pathways. Consequently, these data may suggest that cross-talk be present between PDGFR-α/PI3K and p42/p44 MAPK pathways in mediating the MMP-13 induction by mechanical strain.
Mechanical Strain-induced MMP-13 Expression in a Primary Mouse Osteoblast Cell Model
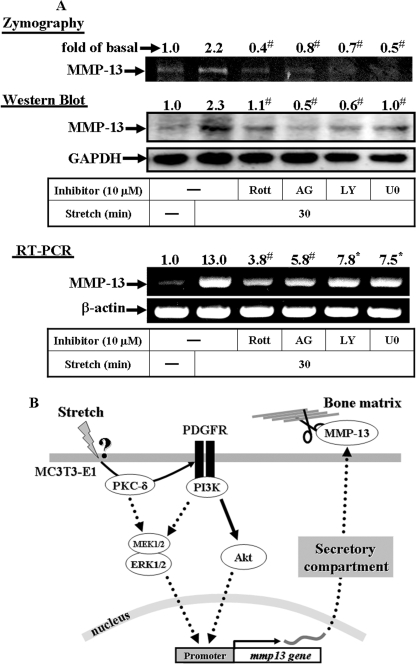
To examine whether a similar pathway regulates MMP-13 expression in normal osteoblastic cells, we obtained osteoblasts from calvaria of neonatal mice through standard protocol of collagenase digestion (19). The resultant MMP-13 expressions with and without specific inhibitors were revealed and analyzed by means of zymogram, Western blot, and RT-PCR (Fig. 8A). The inhibitors include rottlerin, AG1296, LY294002, and U0126 (for MEK1/2). The data showed that all the inhibitors blocked the MMP-13 expression induced by mechanical strain in primary mouse osteoblast cells. To sum up, similar to the MC3T3-E1 cells, at a high impact, the normal osteoblasts respond to the mechanical strain by synthesis and secretion of the MMP-13 and participate in bone healing process.
FIGURE 8.
Effects of inhibitors on MMP-13 expression induced by mechanical strain in primary mouse osteoblasts (A) and schematic summary of the signal transduction mechanism involved in the MMP-13 induction by mechanical strain (B). Osteoblasts were obtained from calvaria of neonatal mice through standard protocol of collagenase digestion (19). The resultant MMP-13 expressions with and without specific inhibitors were revealed by means of zymogram, Western blot, and RT-PCR. The inhibitors include rottlerin, AG1296, LY294002, and U0126 (for MEK1/2), all of which interfere the signaling transduction mechanism leading to the MMP-13 induction by mechanical strain (A). When mechanical stimuli are triggered, certain strained mechano-sensors at the surface of the cell membrane receive the message and initiate a signal transduction mechanism dictating MMP-13 biosynthesis. The signal cascade begins with the PKC-δ molecule by transactivating PDGFR-α and then, the activated PDGFR-α takes over the subsequent signal relay to the MMP-13 expression. The well received PDGFR-α-PI3K-Akt and the known MEK-ERK signaling pathways jointly mediating the MMP-13 gene expression are depicted. The processing of the MMP-13 is independent of the de novo protein synthesis. Accordingly, the MMP-13 synthesis and secretion to the extracellular matrix can be transient and immediate in response to the mechanical stimuli (B).
DISCUSSION
In this study we analyzed the mechanisms by which the mechanical force is translated into biochemical signals and verified the signaling molecules including PKC-δ, PDGFR-α, PI3K, Akt, and ERK1/2 to be responsible for the MMP-13 gene expression. In particular, transfection with shPDGFR-α completely knocked down the PDGFR-α molecule and inhibited MMP-13 zymographic activity and gene induction by mechanical strain. In response to mechanical stimuli, the time-dependent activation of the PDGFR-α and the time-dependent recruitment of the PI3K by PDGFR-α outlined the PDGFR-α/PI3K lineage. Lastly, the signaling relay was verified through sequential phosphorylations.
During our study, we first investigated and considered PDGFR-α to be a candidate receptor for MMP-13 induction by mechanical strain. However, upon continuing to search for the downstream effectors, we found that the known PKC-δ turned out to be the PDGFR-α upstream regulator. Accordingly, the transactivation of PDGFR-α by PKC-δ was proposed and discussed first, whereas the PDGFR-α/PI3K/Akt pathway was discussed later.
PKC activations have been shown to participate in growth factor-dependent cellular responses. For example, the PKC-δ activation is found to be a principle rate-limiting event in the basic fibroblast growth factor-dependent stimulation of MMP-13 in human articular chondrocytes (30), whereas the stimulation of the PDGFR-β signaling pathway activates PKC-δ in the PDGFR-β-mediated-monocytic differentiation (31). Furthermore, the stretch-induced expression of vascular endothelial growth factor appeared to be mediated by the PI3K/PKC-ζ pathway (32). In osteoblasts, PKC activation was reported in the PDGF-BB-induced MMP-13 expression (14). Nevertheless, the specific isoform of the PKCs in this reaction remains unknown. In our stretch system, we identified PKC-δ to be involved in mechanical strain-induced MMP-13 expression. Rather interestingly, instead of being activated by a PDGFR-α-dependent mechanism, PKC-δ activated PDGFR-α. This was supported by the following findings: first, pretreatment with rottlerin inhibited the assembly of the PDGFR-α·PI3K complex and the Akt phosphorylation and second, pretreatment with rottlerin and with dominant negative PKC-δ mutant (ΔPKC-δ) inhibited the PDGFR-α phosphorylation. On the other hand, the results of inhibition by rottlerin or ΔPKC-δ on the mechanical strain-induced p42/p44 MAPK phosphorylation suggested a mechanism of the molecular cross-talk between MAPK and PKC-δ (30). In fact, we also found that cross-talk occurred between PDGFR-α/PI3K and p42/p44 MAPK pathways.
The reports that PDGF-BB induces MMP-13 expression in osteoblasts (14, 15) prompted us to examine the involvement of PDGFR in the mechanical strain-induced MMP-13 expression. There are two classes of PDGF receptors reported to recognize different isoforms of PDGF (33). The β receptor binds only the BB dimer, whereas the α/β receptor binds AA, BB, and AB dimers. In other words, the PDGFR-BB induces all three dimeric combinations of α- and β-receptors. Other reports point out that, in vascular smooth muscle cells, the mechanical strain not only increases PDGF-B and PDGFR-β expression (16) but also activates PDGFR-α (17). In light of these findings, we found that only PDGFR-α participated in the MMP-13 expression induced by mechanical strain in these cells.
Perhaps, one may speculate that mechanical strain changes cellular morphology leading to altered receptor conformation and thus, induces autophosphorylation of the surface membrane receptor and subsequent signal transduction. However, in the identical stretch chamber system, we did not detect any activation of the membrane-associated receptors of PDGFR-β and EGFR examined. On the other hand, we may propose that, being induced by the strain-altered conformational change at the cell membrane, PKC-δ transactivates PDGFR-α. Then, the receptor dimerizes and induces a conformational change affecting the ATP binding domain, which can be blocked by AG1296 at the ATP-binding site (34). One plausible mechanism involved in PKC-δ may be illustrated by the G-protein-coupled receptor-induced EGFR transactivation (35), a ligand-independent process with several cytoplasmic players acting as mediators of this inter-receptor cross-talk. Whether cross-talk exists between PDGFR-α and G-protein-coupled receptor after strain-altered conformational change in our stretch chamber system remains to be defined.
Outside the PDGFR kinase domain (as in kinase insert region or in the C-terminal domain) lie the tyrosine-phosphorylated residues creating the docking sites for signal transduction molecules containing SH2 domains. These molecules may belong to enzymes such as PI3K and phospholipase Cγ (36), or molecules, devoid of enzymatic activity, functioning as an adaptor such as Grb2 in vascular smooth muscle cells linking the receptor with downstream catalytic molecules (17). Despite our efforts to immunoprecipitate the complexes by using anti-PDGFR-α or anti-Grb2 serum as a primary antiserum, we failed to detect the presence of both PDGFR-α and Grb2 within the precipitated complex (data not shown). Nevertheless, when turning toward the enzymatic candidate such as PI3K, we established the PDGFR-α/PI3K connection by a coimmunoprecipitation method. The aggregation was further confirmed by the subsequent experiments of using inhibitors to interrupt the complex formation.
The mechanism of the PDGFR-α/PI3K signaling for MMP-13 induction by mechanical strain could be outlined (Fig. 8B). The transactivation of PDGFR-α by PKC-δ induces dimerization of the PDGFR, leading to their activation via autophosphorylation of tyrosine residues in the PDGFR kinase domain. The autophosphorylation creates docking sites for the recruitment of PI3K. Blocking the kinase activity of PKC-δ would not create docking sites out of the inactive PDGFR-α. Accordingly, no subsequent recruitment of PI3K would occur. Because PDGFR-α binds to p85 regulatory subunit of PI3K, whereas the inhibitor LY294002 inhibits catalytic subunit of PI3K, it is logical that, in the presence of LY294002, PDGFR-α still binds PI3K (37). Moreover, experiments with LY294002 (inhibitor specific for PI3K) suggested that p42/p44 MAPK phosphorylation was downstream to PI3K activation, instead of Akt activation. Transfection with dominant negative mutant Akt had no significant effects on p42/p44 MAPK phosphorylation, indicating no interaction between Akt and p42/p44 MAPK. Thus, we concluded that cross-talk occurred between pathways of p42/p44 MAPK and of PDGFR-α/PI3K (instead of PDGFR-α/PI3K/Akt).
The PDGFR-α has been demonstrated to play an essential, cell-autonomous role in the development of cranial and cardiac neural crest cells (38, 39). Moreover, PDGFR-α has been reported to maintain at a relatively constant level throughout stages of osteoprogenitor and pre-osteoblast (40). It was, however, up-regulated late in the differentiation stage and decreased in relative concert with osteoblast-associated markers such as bone sialoprotein and osteocalcin in the more mature cells. Because PDGFR-α, present in large quantities over longer duration, binds all combinations of PDGF-A/-B forms, one may infer that PDGFR-α meet broader demands for a widespread biological function throughout osteoblast development. Hence, it appears to be natural for the “strain” signal to adopt PDGFR-α in mediating MMP-13 expression.
Bone remodeling to repair the micro-crack, micro-damage, or fatigue damage resulting from the habitual or functional demand has been extensively discussed and reported (1, 3, 6, 41–43). Nevertheless, the bony repair designed by the intentional “stress fracture healing” receives less attention even though such protocols have been implemented for some time in our patient care. The therapeutic means of delivering intermittent heavy mechanical loading includes distraction osteogenesis in patients with Pierre Robin syndrome, rapid maxillary expansion, and orthodontic tooth movement (44) with or without dento-alveolar corticotomy. Even a static application of the high mechanical strain can be illustrated by the surgical bilateral sagittal split osteotomy in patients with mandibular disadvantages (45). All of these operational procedures with high impact loadings demand the immediate response of bone healing/remodeling.
At a high impact, how do osteoblasts adapt and respond at the molecular level? Our working model of transient MMP-13 expression by applying 8% stretch to osteoblasts might explore primitively the underlying mechanism of bone repair. These strategies include the adoptions of the following: 1) the MMP-13 molecule, extracellularly, to be responsible for the degradation of the native collagen in bone remodeling (10), and intracellulary, acting as an immediate early response gene (9), similar to the c-fos gene induction by mechanical stretch in cardiac muscle cells (22); 2) the PDGFR-α molecules, to be abundant and active throughout developments of osteoblasts (40); 3) the PDGFR transactivation model by PKC-δ, similar to the PDGFR transactivation in vascular smooth muscle cells, yet via the G-protein-coupling receptor-mediated mechanism (46); and 4) the PI3K molecule, expressed as a critical control point for the PDGFR/PI3K/Akt signaling in determining the differences of EGF and PDGF in stimulating human mesenchymal stem cells differentiation (47). By integrating these existing modules to operate the synthesis and secretion of MMP-13, osteoblasts respond promptly to meet the therapeutic demands for repair in bone remodeling.
Acknowledgment
We thank Dr. Arthur Veis of Northwestern University for critical reading of the manuscript.
This work was supported by Grant CMRPG331103 (to C. B. W.) from Chang Gung Memorial Hospital, Taiwan and by Grants NSC-96-2320-B-182-003 (to C. M. Y.) and NSC-95-2314-B-002-212 (to C. C. Y.) from National Science Council, Taiwan.
- MMP-13
- matrix metalloproteinase-13
- PDGFR
- platelet-derived growth factor receptor
- PKC
- protein kinase C
- MAPK
- mitogen-activated protein kinase
- ERK
- extracellular signal-regulated kinase
- PI3K
- phosphoinositide 3-kinase
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- α-MEM
- α-minimal essential medium
- RT
- reverse transcriptase
- shPDGFR
- short hairpin PDGFR
- Ffl
- firefly luciferase
- EGFR
- epidermal growth factor
- MEK
- MAPK/ERK kinase.
REFERENCES
- 1.Burr D. B. (1993) Calcif. Tissue Int. 53, S75–S81 [DOI] [PubMed] [Google Scholar]
- 2.Frost H. M. (1998) Calcif. Tissue Int. 62, 89–94 [DOI] [PubMed] [Google Scholar]
- 3.Lanyon L. E. (1996) Bone 18, 37S–43S [DOI] [PubMed] [Google Scholar]
- 4.Somjen D., Binderman I., Berger E., Harell A. (1980) Biochim. Biophys. Acta 627, 91–100 [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa S., Sato S., Saito S., Suzuki Y., Brunette D. M. (1985) Calcif. Tissue Int. 37, 431–436 [DOI] [PubMed] [Google Scholar]
- 6.Jones D. B., Nolte H., Scholübbers J. G., Turner E., Veltel D. (1991) Biomaterials 12, 101–110 [DOI] [PubMed] [Google Scholar]
- 7.Lambert C. A., Soudant E. P., Nusgens B. V., Lapière C. M. (1992) Lab. Invest. 66, 444–451 [PubMed] [Google Scholar]
- 8.Liedert A., Kaspar D., Blakytny R., Claes L., Ignatius A. (2006) Biochem. Biophys. Res. Commun. 349, 1–5 [DOI] [PubMed] [Google Scholar]
- 9.Yang C. M., Chien C. S., Yao C. C., Hsiao L. D., Huang Y. C., Wu C. B. (2004) J. Biol. Chem. 279, 22158–22165 [DOI] [PubMed] [Google Scholar]
- 10.Nagase H., Woessner J. F., Jr. (1999) J. Biol. Chem. 274, 21491–21494 [DOI] [PubMed] [Google Scholar]
- 11.Visse R., Nagase H. (2003) Circ. Res. 92, 827–839 [DOI] [PubMed] [Google Scholar]
- 12.Gack S., Vallon R., Schmidt J., Grigoriadis A., Tuckermann J., Schenkel J., Weiher H., Wagner E. F., Angel P. (1995) Cell Growth & Differ. 6, 759–767 [PubMed] [Google Scholar]
- 13.Ståhle-Bäckdahl M., Sandstedt B., Bruce K., Lindahl A., Jiménez M. G., Vega J. A., López-Otín C. (1997) Lab. Invest. 76, 717–728 [PubMed] [Google Scholar]
- 14.Varghese S., Delany A. M., Liang L., Gabbitas B., Jeffrey J. J., Canalis E. (1996) Endocrinology 137, 431–437 [DOI] [PubMed] [Google Scholar]
- 15.Rydziel S., Durant D., Canalis E. (2000) J. Cell. Physiol. 184, 326–333 [DOI] [PubMed] [Google Scholar]
- 16.Ma Y. H., Ling S., Ivest H. E. (1999) Biochem. Biophys. Res. Commun. 265, 606–610 [DOI] [PubMed] [Google Scholar]
- 17.Hu Y., Böck G., Wick G., Xu Q. (1998) FASEB J. 12, 1135–1142 [DOI] [PubMed] [Google Scholar]
- 18.Heldin C. H., Westermark B. (1999) Physiol. Rev. 79, 1283–1316 [DOI] [PubMed] [Google Scholar]
- 19.Buxton P. G., Bitar M., Gellynck K., Parkar M., Brown R. A., Young A. M., Knowles J. C., Nazhat S. N. (2008) Bone 43, 377–385 [DOI] [PubMed] [Google Scholar]
- 20.Lee A. A., Delhaas T., Waldman L. K., MacKenna D. A., Villarreal F. J., McCulloch A. D. (1996) Am. J. Physiol. 271, C1400–C1408 [DOI] [PubMed] [Google Scholar]
- 21.Karjalainen H. M., Sironen R. K., Elo M. A., Kaarniranta K., Takigawa M., Helminen H. J., Lammi M. J. (2003) Biorheology 40, 93–100 [PubMed] [Google Scholar]
- 22.Sadoshima J., Jahn L., Takahashi T., Kulik T. J., Izumo S. (1992) J. Biol. Chem. 267, 10551–10560 [PubMed] [Google Scholar]
- 23.Vincent T. L., Hermansson M. A., Hansen U. N., Amis A. A., Saklatvala J. (2004) Arthritis Rheum. 50, 526–533 [DOI] [PubMed] [Google Scholar]
- 24.Henriet P., Rousseau G. G., Eeckhout Y. (1992) FEBS Lett. 310, 175–178 [DOI] [PubMed] [Google Scholar]
- 25.Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 26.Tseng C. P., Huang C. L., Huang C. H., Cheng J. C., Stern A., Tseng C. H., Chiu D. T. (2003) FEBS Lett. 541, 21–27 [DOI] [PubMed] [Google Scholar]
- 27.Huang C. L., Cheng J. C., Liao C. H., Stern A., Hsieh J. T., Wang C. H., Hsu H. L., Tseng C. P. (2004) J. Biol. Chem. 279, 42279–42289 [DOI] [PubMed] [Google Scholar]
- 28.Wang W., Zhuang H., Levitz C. L., Fan H., Seldes R. M., Tahernia A. D., Brighton C. T. (1997) Biochem. Mol. Biol. Int. 43, 339–346 [DOI] [PubMed] [Google Scholar]
- 29.Chaudhary L. R., Hruska K. A. (2001) J. Cell. Biochem. 81, 304–311 [PubMed] [Google Scholar]
- 30.Im H. J., Muddasani P., Natarajan V., Schmid T. M., Block J. A., Davis F., van Wijnen A. J., Loeser R. F. (2007) J. Biol. Chem. 282, 11110–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W., Yu J. C., Michieli P., Beeler J. F., Ellmore N., Heidaran M. A., Pierce J. H., Michiel P. (1994) Mol. Cell. Biol. 14, 6727–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuma I., Suzuma K., Ueki K., Hata Y., Feener E. P., King G. L., Aiello L. P. (2002) J. Biol. Chem. 277, 1047–1057 [DOI] [PubMed] [Google Scholar]
- 33.Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. (1988) Science 240, 1529–1531 [DOI] [PubMed] [Google Scholar]
- 34.Kovalenko M., Rönnstrand L., Heldin C. H., Loubtchenkov M., Gazit A., Levitzki A., Böhmer F. D. (1997) Biochemistry 36, 6260–6269 [DOI] [PubMed] [Google Scholar]
- 35.Prenzel N., Zwick E., Leserer M., Ullrich A. (2000) Brest Cancer Res. 2, 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Y. H., Reusch H. P., Wilson E., Escobedo J. A., Fantl W. J., Williams L. T., Ives H. E. (1994) J. Biol. Chem. 269, 30734–30739 [PubMed] [Google Scholar]
- 37.Vlahos C. J., Matter W. F., Hui K. Y., Brown R. F. (1994) J. Biol. Chem. 269, 5241–5248 [PubMed] [Google Scholar]
- 38.Soriano P. (1997) Development 124, 2691–2700 [DOI] [PubMed] [Google Scholar]
- 39.Tallquist M. D., Soriano P. (2003) Development 130, 507–518 [DOI] [PubMed] [Google Scholar]
- 40.Liu F., Malaval L., Aubin J. E. (2003) J. Cell Sci. 116, 1787–1796 [DOI] [PubMed] [Google Scholar]
- 41.Martin R. B., Burr D. B. (1989) Structure, Function, and Adaptation of Compact Bone, pp. 186–213, Raven Press, New York [Google Scholar]
- 42.Frost H. M. (1994) Angle Orthodontist 64, 175–188 [DOI] [PubMed] [Google Scholar]
- 43.Frost H. M. (1997) J. bone Miner. Res. 12, 1539–1546 [DOI] [PubMed] [Google Scholar]
- 44.Chang H. H., Wu C. B., Chen Y. J., Weng C. Y., Wong W. P., Chen Y. J., Chang B. E., Chen M. H., Yao C. C. (2008) J. Dent. Res. 87, 692–696 [DOI] [PubMed] [Google Scholar]
- 45.Lee Y. T., Chen M. C., Chen H. L., Wu C. B. (2009) Chang Gung Med. J. 32, 320–329 [PubMed] [Google Scholar]
- 46.Tanimoto T., Lungu A. O., Berk B. C. (2004) Circ. Res. 94, 1050–1058 [DOI] [PubMed] [Google Scholar]
- 47.Kratchmarova I., Blagoev B., Haack-Sorensen M., Kassem M., Mann M. (2005) Science 308, 1472–1477 [DOI] [PubMed] [Google Scholar]