Abstract
Background and Purpose
Changes in shear and medial wall stress induced by blood flow contribute to vascular remodeling, but details of these relations remain undefined. We hypothesized that remodeling has a strong genetic component and that phenotypic responses to hemodynamic stress will differ among rat strains. Here, we characterized phenotypic traits related to carotid remodeling in the 2 rat strains that we previously showed the greatest difference in shear stress regulation: Genetically Hypertensive (GH) and Brown Norway (BN) rat strains.
Methods
Left internal and external carotid arteries were ligated and blood flow was reduced in the left common (LCA) by 90% and increased in the right common carotid artery (RCA) by 60%. Rats were studied for up to 28 days following flow modification and carotid outer diameters were measured in vivo, and wall and luminal components by histomorphometry, to obtain indices of remodeling. Blood flow and pressure measurements were made at corresponding time-points.
Results
By day 28, remodeling in the GH was greater in response to high flow than in BN, and shear stress was normalized. In contrast, remodeling in the BN was greater in the low flow LCA than in GH. Media stress was greater in GH than BN for any value of carotid shear stress and remained relatively unchanged in low flow, but markedly increased in high flow remodeling. Importantly, pressure was not a major determinant of flow-remodeling in these conditions.
Conclusions
There are key differences in the ability of carotids in GH and BN rats to adhere to hemodynamic laws during vascular remodeling. GH rats exhibit intact regulatory mechanisms for increased, but not reduced shear stress. Moreover, the ability to maintain physiological shear and media stresses during vascular remodeling in response to modified flow appears to be intrinsically “genetically” determined.
Keywords: Carotid artery, blood flow, hypertension, vascular remodeling, shear stress, media stress, rats
Introduction
Vascular remodeling is a complex pathophysiological process that plays an important role in the clinical manifestation of cardiovascular disease, including ischaemic stroke. Engineering principles based on the laws of Poiseuille and Laplace illustrate the mechanical effects of fluid movement through hollow pipes. Poiseuille's law states that volume flow rate is determined by the pressure difference divided by the viscous resistance (Q=ΔPπr4/8ηl; where ΔP is pressure, r is lumen radius, η is viscosity and l is length). For blood vessels (assuming circular lumens with parabolic velocity profiles) one can determine the dragging frictional force, termed shear stress (τ), exerted by blood on the vessel wall. Based on Poiseuille's equation (τ=4ηQ/πr3) it is clear that small changes in radius result in marked changes in shear stress. Thus narrowing of the radius will cause a local increase in shear stress. Laplace's law (S=Pr/h) states that the wall tensile stress (S) is directly proportional to pressure (P) and radius (r) and inversely related to wall thickness (h). Thus increases in pressure are compensated for by increased wall thickness to maintain wall stress constant. Blood vessels, however, are more complex than pipes since they can dynamically respond rapidly (tone) and chronically (remodeling) to mechanical forces in accordance with these laws.
It is presently clear that hypertension is a major risk factor for atherosclerosis (strong correlation between fatty streak formation and systolic blood pressure) and for clinical events (strong correlation between unstable angina, myocardial infarction, stroke and systolic blood pressure). It is considered that thickening of the vessel wall to normalize wall tension is the primary biological signal driving remodeling in hypertension1,2. Wall thickening (in the absence of compensatory outward remodeling) may cause inward remodeling and exacerbate the clinical symptoms associated with coronary and carotid stenoses 3. A further complication of wall thickening associated with hypertension is increased vascular stiffness due to modulation of extracellular matrix synthesis4. Hypertension may therefore limit compensatory outward remodeling that occurs in atherosclerosis and restenosis because the expected response to increased pressure is inward remodeling. The degree of vascular remodeling observed in patients with atherosclerosis is highly variable5 and this suggests that pressure-independent genes have a key role to play in remodeling, too. This concept is further supported by the observation that the rate of restenosis (due to remodeling) in hypertensive patients remains high despite blood pressure control6. Thus, hypertension, a potent promoter of atherosclerosis may increase the incidence of occlusive stenosis by a negative effect on compensatory remodeling. Understanding the interactions between hypertension, atherosclerosis and vascular remodeling is therefore likely to have important clinical implications.
Evidently, prolonged alterations in mechanical stresses can modulate vascular structure but many aspects of these relationships remain unclear. It is recognized that there are alterations in the endothelium, smooth muscle cells and extracellular matrix in hypertension which may modulate remodeling since this process depends on participation of both the cellular and extracellular components of the vascular wall. Previously, we reported striking differences in outward and inward remodeling responses between the New Zealand strain of GH and BN rats7 but whether there are differences in regulation of shear and wall stress needs to be better understood. We hypothesized that remodeling has a strong genetic component and that phenotypic responses to hemodynamic stress will differ among rat strains. Here, we characterized phenotypic traits related to carotid remodeling in the 2 rat strains that we previously showed the greatest difference in shear stress regulation: Genetically Hypertensive (GH) and Brown Norway (BN) rat strains.
Materials and Methods
The investigation conforms with Animal care and experimental procedures described were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All aspects of this study were approved by the University of Rochester Animal Care Committee. Rats were bred in-house (brother-sister mating to maintain genetic homozygosity) in the animal facilities.
Implantation of radio-telemetric blood pressure probes
At 5 weeks, male rats were anaesthetized with intra-peritoneal ketamine hydrochloride (5mg/100g), xylazine (0.5mg/100g), and acepromazine maleate (0.5mg/100g). The abdominal aorta was isolated and the catheter of the radiotelemetry pressure probe (TA11PA-C20) was inserted. BP was measured every 5mins, each recording representing the average of pressure measurements made during ~60 heart-beat periods over 10 sec intervals. Five minute recordings were averaged to generate hourly measurements and then used to determine 24hour pressures.
Chronic alteration of blood flow in vivo by arterial ligation
At the age of 7 weeks, rats were re-anaesthetized (using the same anaesthesia as above) and LCA, RCA and the distal branches of the LCA (left internal and external CA) were isolated, as described previously7. Sham-operated (surgery without ligations) rats were also studied. Measurements of LCA and RCA outer diameter (OD) were made with a dissecting microscope reticule. We previously showed a highly significant correlation between measurements made by reticule and by histology, although OD is 20% smaller by histology due to tissue shrinkage during fixation8. Blood flow (BF) was measured with a Transit-time ultrasonic flow probe, once BF had stabilized.
LCA branches were ligated, whilst ensuring patency of the occipital artery. The thyroid artery was also ligated if its origin was proximal to the occipital artery. BF and OD measurements were repeated. Rats were maintained on a standard rodent diet and water ad libitum.
At 3, 7 or 28 days post-ligation, rats were re-anaesthetized and the CA isolated. BF and OD measurements were repeated. The ΔOD (OD at 4 weeks - OD initial) and Δ shear stress (shear stress at 4 weeks - shear stress initial) were determined to account for differences at baseline; (Δflow was similarly determined). Thus, substantial outward remodeling in response to increased flow is associated with a high ΔOD, and substantial inward remodeling in response to decreased flow is associated with a low ΔOD. On termination, abdominal aortae were isolated and catheterized to enable retrograde perfusion. Rats were perfusion-fixed at mean BP with 10% formalin.
Histomorphometric analyses
Carotids were harvested, processed for histology and 4μm paraffin-embedded cross-sections were stained with haematoxylin and eosin and Verhoeff-van Gieson. Computer assisted image analysis was performed, as described7.
Shear and media stress determination
Shear stress was calculated according to the Poiseuille's equation: τ= 4ηQ/πr3, where η is the viscosity of blood (assumed constant in both strains), Q is the BF (ml/sec), and r is the vessel radius (cm). Media tension was calculated in accordance with Laplace's Law from SBP x r, where SBP is systolic blood pressure (dynes/cm2) and r is the internal carotid radius (cm) determined histomorphometrically. Media stress was calculated from media tension/h, where h is medial thickness (cm), determined histomorphometrically.
Statistical Analysis
Results are reported as mean ± SEM. Differences between groups were analyzed by ANOVA followed by Fisher's post-hoc tests. Student's paired t-test was used for comparisons at baseline and termination within rat strains. Correlations were determined by multiple and simple linear regression analyses; quality of regression was assessed by Pearson's correlation coefficient. P<0.05 was regarded as significant. Statistical tests were performed with StatView for MacIntosh, version 5.01.
Results
Significant differences in GH and BN rat SBP, flow, OD and shear stress were found at baseline (Supplement Tables 1A and 1B show data at day 0 for all times and groups) and consequently % change at harvest times (shown in figures) was used to compare strains. Weight gain in both strains was similar. At 28 days, weight was increased by ~50% from baseline in GH and BN rats.
Blood flow, outer diameter, shear stress and flow-induced vascular remodeling: real time measurement
For the experiments described below we measured flow and outer diameter (OD) simultaneously. Acutely following LCA ligation, flow in the RCA increased by ~45%, and in the LCA decreased by ~90% in both strains. Note that the initial change in BF did not differ between strains.
By day 28 GH LCA flow remained ~90% reduced, but BN LCA flow progressively increased to ~40% less than sham carotid flow (Figure 1A). The time-course of LCA OD responses to decreased flow in GH rats showed a decline to a minimum within 7 days but thereafter rapidly recovered over the next 3 weeks (Figure 1B). BN LCA remodeled inward to a significantly greater extent over 28 days than the GH LCA as shown by a negative change in OD.
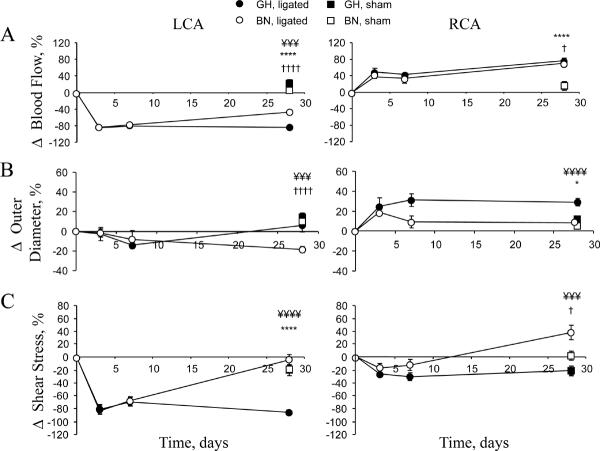
Figure 1. Time-course of flow, outer diameter and shear stress changes in LCA and RCA.
(A) Change in blood flow in LCA and RCA. At 28 days in the RCA, flow was 4.62±0.17 ml/min and 5.33±0.21 ml/min in GH and BN rats, respectively. At 28 days, flow in the LCA was 0.43±0.05 ml/min and 1.51±0.09 ml/min in GH and BN rats, respectively. (B) Change in outer diameter measured in anesthetized animals. OD at 28 days in the RCA was 1.30±0.03 mm and 1.09±0.03 mm in GH and BN rats, respectively. At 28 days, LCA OD was 0.99±0.04 mm and 0.84±0.03 mm in GH and BN rats respectively. (C) Change in shear stress (SS). RCA SS at 28 days was 12.79±0.96 dynes/cm2 and 25.65±2.13 dynes/cm2 in GH and BN rats, respectively. At 28 days, LCA SS was 2.72±0.39 dynes/cm2 and 16.17±1.71 dynes/cm2 in GH and BN rats, respectively. For ease of presentation statistical significance is indicated in all figures as *P<0.05, **P<0.01, ***P<0.005, ****P<0.001 for GH ligated versus GH sham; †P<0.05, ††P<0.01, ††††P<0.001 for BN ligated versus BN sham; ¥P<0.05, ¥¥P<0.01, ¥¥¥P<0.005, ¥¥¥¥P<0.001 for GH ligated versus BN ligated. Note there were no significant differences between BN and GH shams. Analysis used 2-way ANOVA (factors rat strain and intervention) followed by Fisher's post-hoc tests for the 28 day data points, as described.
Shear stress values followed the OD results with normalization of shear stress in the BN LCA by 28 days, while the GH LCA showed a marked reduction in shear stress (Figure 1C) since the OD increased (Figure 1B).
Chronic RCA flow responses to ligation exhibited a similar increase (~75%) in GH and BN RCA (Figure 1A). RCA OD responses to increased flow showed a marked change within 3 days of ligation in both strains (Figure 1B). The rate of increase was greater in GH RCA, peaking at 7 days and then sustained for the following 3 weeks. To evaluate whether the changes at 3 days were structural or functional (change in tone) we applied nitroglycerin externally, and observed no change in OD. In BN rats, the 3 day peak increase in RCA OD subsequently declined to a minimum by 7 days. Thus, despite similar changes in flow, the GH RCA remodeled outward to a much greater extent than the BN RCA. Shear stress values followed the OD results with normalization of shear stress in the GH RCA, while the BN RCA showed a marked increase in shear stress since the outer diameter did not change (Figure 1C). Thus the remodeling responses were opposite for both high and low flow in BN and GH rats. These data suggest strain-specific differences in the remodeling responses to changes in blood flow.
Histomorphometric analyses of remodeled vessels
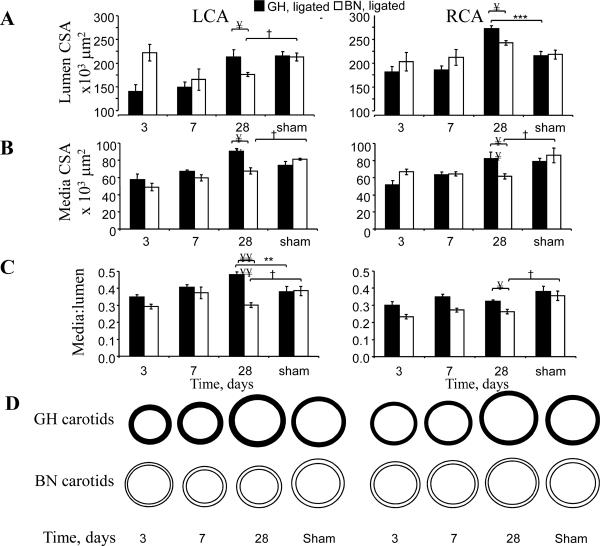
To determine the rates of change in carotid wall components in GH and BN rats, we performed a time-course of histomorphometric measurements (Figure 2A-D). There were significant changes in lumen and media areas of LCA and RCA in both strains, but the differences contrasted significantly between strains. Specifically, lumen areas increased in GH LCA over time so that by day 28 they did not differ from shams whereas BN LCA lumen areas progressively declined to 17% below that of sham LCA (Figure 2A). Media cross-sectional areas in response to flow reduction exhibited a steep time-dependent increase in GH LCA that was greater than in BN LCA (Figure 2B, Supplement Table 2). By 4 weeks of flow reduction, there was an 8% increase in media cross-sectional area in GH LCA in contrast to the 24% decrease in BN LCA, compared to respective sham-operated arteries. These results support our previous studies in mice that increases in media area correlate with increases in lumen area9.
Figure 2. Histomorphometric analyses of LCA and RCA.
A. Lumen cross sectional area (CSA). B. Media CSA. C. Media/lumen ratios. D. Schematic representation of remodeling data. Note all data from paraffin embedded and formalin fixed sections. Analysis used 2-way ANOVA (factors rat strain and intervention) followed by Fisher's post-hoc tests for the 28 day data points, as described.
In accordance with the rapid increase in media cross-sectional area in GH LCA, the most dramatic findings were that the LCA media:lumen ratio by 28 days increased by 28% in GH, but decreased by 18% in BN rats, compared with respective sham-carotids (Figure 2C). The effect of flow reduction on adventitia cross-sectional area at 4 weeks was negligible in both rat strains relative to LCA from sham-operated rats.
RCA lumen and media areas exhibited three major differences between GH and BN rats (Figure 2A-D). First, although the lumen areas of both GH and BN increased compared to shams, GH lumen areas exhibited a greater rate and magnitude of increase. Second, by day 28 the media area of BN decreased compared to sham, while the GH RCA media area did not change from GH sham resulting in a highly significant difference between GH and BN media areas. Consistent with these results the media:lumen ratio was significantly decreased in BN RCA compared to sham. There were modest increases in adventitial cross-sectional area of 3% and 6% in GH and BN RCA, respectively, compared with sham carotids at 28 days (not shown).
Thus, histomorphometric analyses of carotids reveals that in response to 4 weeks of reduced flow, GH LCA exhibited hypertrophic remodeling (as defined by Mulvany1) whereas BN LCA exhibited inward hypotrophic remodeling (Figure 2D). In response to increased flow for 4 weeks, RCA exhibited outward eutrophic remodeling in GH but outward hypotrophic remodeling in BN rats.
Blood pressure and flow-induced vascular remodeling
There were significant systolic BP (SBP) differences between GH and BN rats, as expected. At baseline in GH and BN rats, SBP was 155±3 vs. 107±2 mmHg (P<0.0001), and 28 days after ligation SBP was 180±2 vs. 106±8 mmHg (P<0.0001), respectively. Similarly, pulse pressure (PP) at baseline was 59±1 vs. 32±1 mmHg (P<0.0001), and 28 days after ligation surgery was 68±1 vs. 31±2 mmHg (P<0.0001) in GH and BN rats, respectively.
Correlation analyses of systolic, diastolic, and mean blood pressure, pulse pressures, and OD were performed to determine if they were determinants of carotid OD. No significant effects of blood pressure variables on RCA or LCA OD were found indicating that pressure did not significantly affect remodeling.
We performed a multivariate regression analysis with BP variables and rat strain as the independent factors, and OD as the dependent variable. This identified strain as the significant determinant of OD. Subsequently, we also performed correlation analyses (simple regression) for each strain. Correlation analyses of systolic, diastolic, mean blood pressure, and pulse pressures with OD were performed to determine if they were determinants of carotid OD. Both absolute values and the change in BP variables were analyzed. Both the low flow and high flow changes were also analyzed. No significant effects of blood pressure variables on RCA or LCA OD were found indicating that pressure did not significantly affect remodeling. Note that at 28 days the GH SBP range was 147-188 mm Hg and the BN SBP range was 77-129 mm Hg so that the spread of BPs within each strain was sufficient to yield a positive correlation if present. Thus, our analysis of the correlations between BP variables and remodeling indices indicate that rat strain (and hence genetic differences), not BP, was the determinant of the remodeling response.
Media tension, media stress and flow-induced vascular remodeling
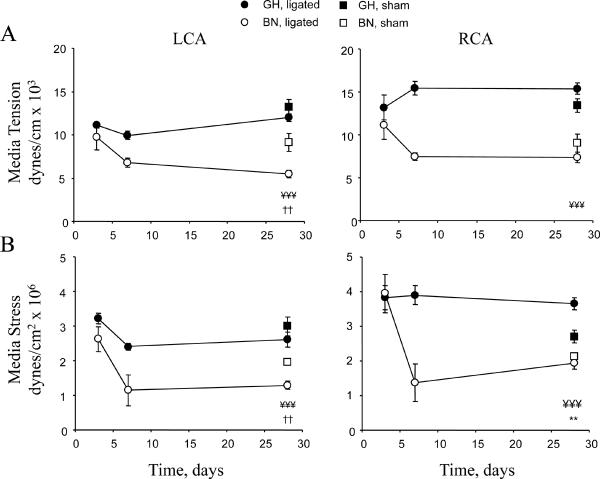
Because media tension and stress are also important determinants of vascular remodeling 1,2, we determined their relationship (Figure 3). Carotid media stress was derived by normalizing media tension to media thickness. At the time of carotid ligation media tension (~12dynes/cm × 103) was similar in RCA and LCA of both strains. Media stress at ~3.5dynes/cm2 × 106 was also similar. However, after 28 days there were significant differences in tension and stress. To summarize the data, media tension and stress were lower in normotensive BN than hypertensive GH in both sham and ligated animals. In low flow LCA, media tension in BN was significantly decreased compared to GH (Figure 3A). The ligated BN LCA was lower than the BN sham also, while there was no change in GH compared to sham (Figure 3A). Changes in media stress were similar to media tension (Figure 3B). In high flow RCA, media tension was strikingly different between GH and BN at 28 days with significantly higher tension in GH than BN. In GH RCA, media stress was significantly higher than sham carotids and BN, while BN RCA stress was the same as BN shams (Figure 3B).
Figure 3. Time-course of media tension and stress.
(A) Media tension in LCA and RCA. (B) Media stress in LCA and RCA. Analysis used 2-way ANOVA (factors rat strain and intervention) followed by Fisher's post-hoc tests for the 28 day data points, as described.
To evaluate the influence of SBP on carotid media stress, we performed a multivariate regression analysis as above, followed by correlation analyses. Multivariate regression models for RCA and LCA media stress with SBP and rat strain as the independent factors, identified strain (P<0.05) as the significant determinant, suggesting intrinsic differences in the responses to high and low flow. In high flow RCA and low flow LCA, media stress and SBP were not correlated in either rat strain, when these variables were analyzed individually in each strain.
Regulation of shear stress
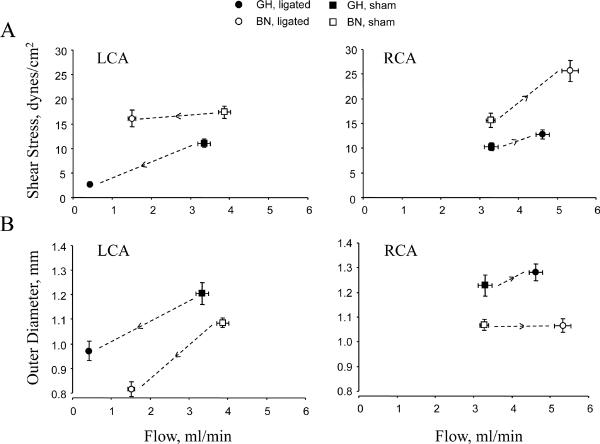
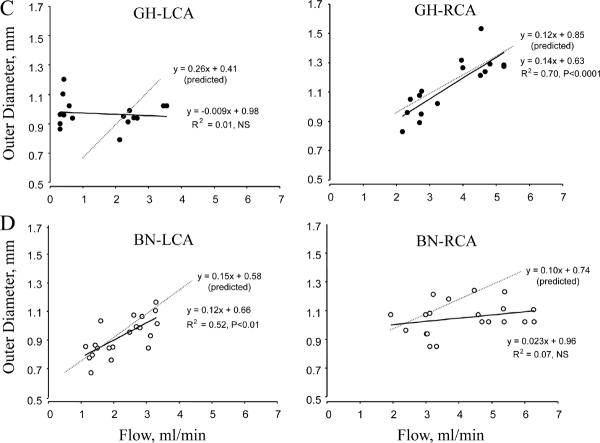
To gain insight into the mechanical influences of flow on carotid structure, we performed correlation analyses of (1) flow and shear stress, and (2) flow and OD. In low flow LCA, shear stress was maintained constant in BN while it decreased in GH vessels (Figure 4A). This correlated with a greater decrease in OD for a given change in flow (Figure 4B) suggesting that BN carotids were more sensitive than GH to a reduction in flow. In the RCA the remodeling was opposite, with a greater increase in shear stress in BN than GH vessels (Figure 4A) suggesting reduced sensitivity to the change in flow. This was paralleled by no change in OD of BN vessels (Figure 4B). To further investigate remodeling in GH and BN, flow and carotid OD scatterplots were constructed (Figure 4C-D), and the theoretical flow and OD relations necessary to maintain constant shear stress were also determined. In low flow LCA, the measured flow and OD relation in BN did not differ from the theoretical relation but GH LCA deviated significantly. Conversely, in high flow RCA, BN deviated significantly from the predicted relation between flow and OD but GH RCA responded appropriately.
Figure 4. Shear stress and outer diameter as a function of flow in LCA and RCA.
(A) Shear stress vs. flow. (B) OD vs. flow. Arrows in A. and B. depict direction of change from shams. (C-D) Scatterplots depict OD as a function of flow. Predicted flow and OD relations (dotted gray lines) to maintain constant shear stress are also shown.
In a multivariate regression model with flow and rat strain as the independent factors, strain (P<0.05) was a significant determinant of RCA shear stress, while no factor was a determinant of LCA shear stress. Similar multivariate regression models for RCA and LCA OD identified both strain (P<0.05) and flow (P<0.001) as significant determinants.
Together, these results indicate that (1) shear stress is regulated by threshold-sensitive processes, and (2) shear stress regulation is strain-dependent.
Regulation of media stress
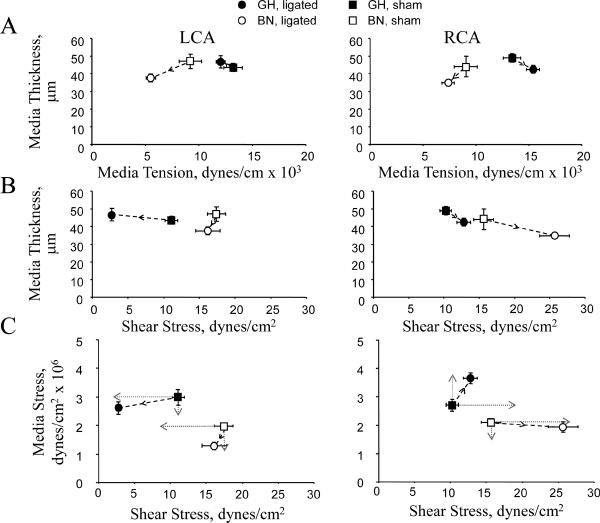
Media thickness is a critical determinant of media stress and we therefore examined two additional relationships to understand mechanical stress-induced vascular remodeling: (1) Media thickness and media tension, and (2) Media thickness and shear stress. In RCA and LCA, GH rats exhibited a negative correlation between media thickness and media tension but BN rats exhibited a positive association (Figure 5A). Thus, tension had a negative effect on media thickness in GH carotids but a positive effect in BN which means that media stress was not normalized in GH but was in BN RCA. Media thickness and shear stress were negatively correlated in GH and BN RCA (Figure 5B). Similarly, there were negative correlations between media thickness and shear stress in the GH LCA. In BN LCA, however, there was no change in shear stress so we cannot define the effect on media thickness. Thus, shear stress negatively affected media thickness in GH RCA and LCA, and BN RCA.
Figure 5. Media thickness and stress related to tension and shear stress.
(A) Media thickness vs. tension. (B) Media thickness vs. shear stress. (C) Media stress vs shear stress. Predicted media and shear stress relations (dotted gray lines) to maintain constant stress and shear stress levels are also shown. Arrows depict direction of change from shams.
In summary, shear stress and media stress are differentially regulated in GH and BN carotids (Figure 5C). In low flow, shear stress was decreased by 80% in GH with little change in media stress; shear stress remained unchanged in BN but media stress was reduced by 40%. In high flow, shear stress remained unchanged in GH but media stress was increased by 35%; in BN there was a 70% increase in shear stress but little change in media stress.
Discussion
Vessels remodel to maintain flow, shear stress, and wall tension at physiological levels. Under pathologic conditions, such as high pressure or decreased flow, the ability of the vessel to maintain all three parameters constant may be compromised. Thus a major finding of this study is that in response to increased blood flow, a model for physiologically beneficial situations such as exercise, carotid arteries of GH rats remodel to an equal or greater extent than those of BN rats. However, in response to low blood flow as occurs in many vascular diseases, GH carotids exhibited an impaired ability to remodel. Importantly, pressure was not a major determinant of flow-remodeling in these conditions. Finally, while media stress was significantly lower in BN than GH carotids, the relative change in media stress in low flow was greater than in GH whereas in high flow the change in media stress was smaller than in GH, suggesting that distinct regulatory mechanisms are involved.
In the high flow RCA, the physiologic response should be to normalize shear stress by increasing lumen area, thereby obeying Poiseuille's law. As the vessel enlarges, the wall tension will increase so media thickness should increase to normalize wall stress. We found that both GH and BN increased outer diameter and lumen area as anticipated (Figures 1B and 2A). However, the BN response was inadequate to normalize shear stress. Because the change in blood flow did not differ between the two strains these results suggest a fundamental difference in sensing or transducing the signals elicited by increased flow, and in the response of the wall components, particularly endothelial and medial cells. Insight into possible mechanisms comes from the observation that while media area was similar in GH and BN shams, flow elevation reduced media area in BN but not in GH. Previously we found a strong linear correlation between the increase in media area and the extent of outward vessel remodeling among 5 strains of mice9. Combined with the results of the present study, our data suggest that mechanisms responsible for media thickening are primary determinants of outward vessel remodeling in response to changes in flow.
In the low flow LCA, a model for pathophysiological situations, the predicted vascular responses would be to decrease lumen diameter to normalize shear stress and to decrease media thickness to normalize wall stress. We found that BN decreased outer diameter and lumen area as expected, while GH did not change significantly from sham. Thus BN appropriately normalized shear stress (but not media stress). In contrast, media stress, but not shear stress was greater in GH than BN (Figure 5). Consistent with our hypothesis regarding media thickening, there was a significant decrease in BN media area with no change in GH (Figure 2B). Thus, it appears that the GH LCA remodels by increasing media area. This may be due to augmented growth mechanisms/restricted sensitivity to apoptotic stimuli. The finding that the GH carotid in low flow regions has the ability to remodel outward is particularly interesting since this has been observed clinically10-12, and in our studies using mouse carotids9,13.
The initiating stimulus for flow-induced remodeling is likely to be shear stress which is subsequently transduced by the vessel wall to produce a structural response. Based on findings that flow-mediated vasodilation in hypertensive humans is impaired14, we anticipated that remodeling in the GH would be impaired. However, we provide evidence to the contrary since hypertension per se did not restrict outward remodeling, and nor did it enhance inward remodeling. The enhanced compensatory response of hypertensive vessels to elevated shear stress may be attributed to growth processes (such as cellular hypertrophy and hyperplasia, protein synthesis and extracellular matrix protein reorganization), which are necessary to enlarge vessels. Hypertensive vessels have augmented intrinsic capacity for cellular growth15-17, and pressure directly upregulates matrix metalloproteinase expression which may facilitate outward remodeling18. These mechanisms may contribute to the maintenance, or increase, in media thickness that we propose is a key mediator of the remodeling response. It is also possible that hypertensive vessels have a differential response to mechanical stimuli, due to differences in wall composition. Thus, examination of diameter-distension curves of remodeled vessels would further understanding of vascular responses to mechanical stimuli.
It appears that the set point for media tension is higher in GH than BN (Figure 5A). After 28 days, both media tension and stress were higher in GH than BN. In fact the difference in media tension of ligated GH and BN RCA (15.3 versus 7.2 dynes/cm × 103, a 112% increase) was the largest difference in any parameter determined. It is intuitive that this difference is a consequence of hypertension. The key finding is that despite failing to normalize wall stress, the GH rat actually remodeled more effectively (greater lumen) than the BN rat. A possible explanation is that the “cost” of responding to increased flow demands media responses that are predominantly growth and hypertrophy rather than apoptosis and hypotrophy, and apparently may not be sufficient even in a hypertensive background.
Taken together, distinct strain and threshold-specific mechanisms involving signal transduction processes are likely involved in regulating local mechanical stresses in GH and BN carotids. Consequently, these stress regulatory patterns will have disparate effects on mechanical and functional properties in vessel walls of these rat strains. Vascular remodeling in disease regions such as atherosclerosis is likely to proceed, in part, by the same pathways as flow-induced remodeling. Hypertension, a potent promoter of atherosclerosis, may be envisaged to increase the incidence of atherosclerosis by reducing the ability of blood vessels to compensate for plaque burden (a process that requires outward vascular remodeling)5. This study, however, suggests that elevated pressure per se does not restrict outward remodeling. While short-term this “approach” to remodeling may be beneficial by increasing the rate and extent of remodeling, there is a price to pay for the elevated media tension and stress in GH vessels. Wall stress is likely detrimental by promoting vascular damage due to fatigue effects, increased resistance and reduced distensibility, and possibly increased intima-media thickness, a recognized cardiovascular risk factor positively associated with increased media stress19.
In summary, there are key differences in the ability of carotids in GH and BN rats exposed to modified flow to adhere to hemodynamic laws during vascular remodeling, indicating that the ability to maintain physiological shear and media stresses is intrinsically “genetically” determined. In particular, inappropriate compensatory adaptation to changes in shear stress is a common feature contributing to vascular disease processes, such as ishaemic stroke. Our findings suggest that the GH and BN vasculature are ideal models for mechanistic studies into failure to regulate low and high shear stresses, respectively. Future studies directed at mechanotransduction in the vascular wall will identify genetic/molecular mechanisms responsible for differential responsiveness to mechanical stresses in these rat strains, and may provide novel approaches in the treatment of vascular disease with critical remodeling components20.
Supplementary Material
Acknowledgements and Funding
The authors acknowledge support from National Institutes of Health (HL-62826 to BCB). We also thank David Nagel for help with histomorphometry.
Footnotes
Conflict of Interest None declared.
References
- 1.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension. 1996;28:505–6. [PubMed] [Google Scholar]
- 2.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 3.Kiechl S, Willeit J. The natural course of atherosclerosis. Part I: incidence and progression. Arterioscler Thromb Vasc Biol. 1999;19:1484–90. doi: 10.1161/01.atv.19.6.1484. [DOI] [PubMed] [Google Scholar]
- 4.Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–8. doi: 10.1161/01.hyp.36.3.312. [DOI] [PubMed] [Google Scholar]
- 5.Glagov S. Intimal hyperplasia, vascular modeling, and the restenosis problem. Circulation. 1994;89:2888–2891. doi: 10.1161/01.cir.89.6.2888. [DOI] [PubMed] [Google Scholar]
- 6.Gurlek A, Dagalp Z, Oral D, Omurlu K, Erol C, Akyol T, Tutar E. Restenosis after transluminal coronary angioplasty: a risk factor analysis. J Cardiovasc Risk. 1995;2:51–5. [PubMed] [Google Scholar]
- 7.Ibrahim J, Miyashiro JK, Berk BC. Shear stress is differentially regulated among inbred rat strains. Circ Res. 2003;92:1001–1009. doi: 10.1161/01.RES.0000069687.54486.B1. [DOI] [PubMed] [Google Scholar]
- 8.Miyashiro JK, Poppa V, Berk BC. Flow-induced vascular remodeling in the rat carotid artery diminishes with age. Circ Res. 1997;81:311–9. doi: 10.1161/01.res.81.3.311. [DOI] [PubMed] [Google Scholar]
- 9.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: the “Glagov phenomenon” is genetically determined. Circulation. 2004;110:220–6. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- 10.Wentzel JJ, Janssen E, Vos J, Schuurbiers JC, Krams R, Serruys PW, de Feyter PJ, Slager CJ. Extension of increased atherosclerotic wall thickness into high shear stress regions is associated with loss of compensatory remodeling. Circulation. 2003;108:17–23. doi: 10.1161/01.CIR.0000078637.21322.D3. [DOI] [PubMed] [Google Scholar]
- 11.Stone PH, Coskun AU, Kinlay S, Clark ME, Sonka M, Wahle A, Ilegbusi OJ, Yeghiazarians Y, Popma JJ, Orav J, Kuntz RE, Feldman CL. Effect of Endothelial Shear Stress on the Progression of Coronary Artery Disease, Vascular Remodeling, and In-Stent Restenosis in Humans. In Vivo 6-Month Follow-Up Study. Circulation. 2003 doi: 10.1161/01.CIR.0000080882.35274.AD. [DOI] [PubMed] [Google Scholar]
- 12.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–93. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 13.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–91. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 14.Pan XM, Nelken N, Colyvas N, Rapp JH. Inhibition of injury induced intimal hyperplasia by saralasin in rats. J Vasc Surg. 1992;15:693–8. [PubMed] [Google Scholar]
- 15.Ibrahim J, Hughes AD, Schachter M, Sever PS. Enhanced tissue polyamine content in the spontaneously hypertensive rat. Clin Exp Pharmacol Physiol. 1996;23:410–4. doi: 10.1111/j.1440-1681.1996.tb02750.x. [DOI] [PubMed] [Google Scholar]
- 16.Ueno H, Kanellakis P, Agrotis A, Bobik A. Blood flow regulates the development of vascular hypertrophy, smooth muscle cell proliferation, and endothelial cell nitric oxide synthase in hypertension. Hypertension. 2000;36:89–96. doi: 10.1161/01.hyp.36.1.89. [DOI] [PubMed] [Google Scholar]
- 17.Berk BC, Vallega G, Muslin AJ, Gordon HM, Canessa M, Alexander RW. Spontaneously hypertensive rat vascular smooth muscle cells in culture exhibit increased growth and Na+/H+ exchange. J Clin Invest. 1989;83:822–829. doi: 10.1172/JCI113964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehoux S, Lemarie CA, Esposito B, Lijnen HR, Tedgui A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation. 2004;109:1041–7. doi: 10.1161/01.CIR.0000115521.95662.7A. [DOI] [PubMed] [Google Scholar]
- 19.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–9. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 20.Berk BC, Korshunov VA. Genetic determinants of vascular remodelling. Can J Cardiol. 2006;22(Suppl B):6B–11B. doi: 10.1016/s0828-282x(06)70980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.