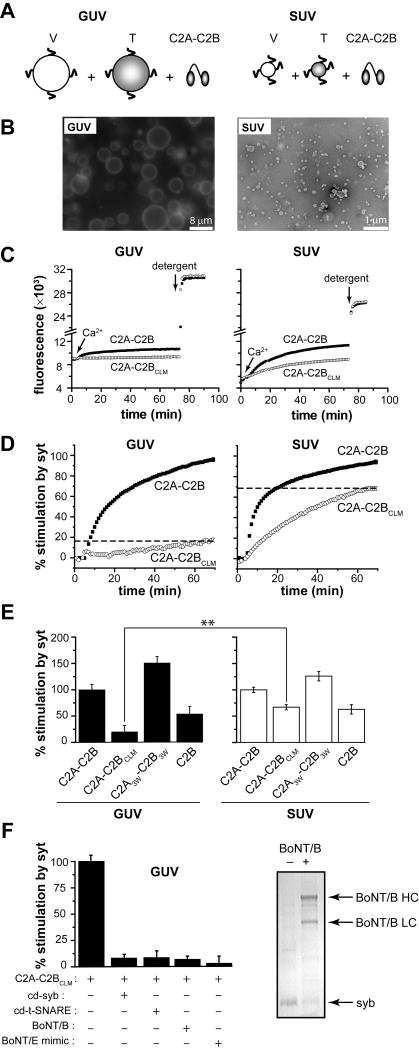
Figure 4. A tubulation-deficient syt mutant regulates the fusion of small, but not large, liposomes.
(A) Syt-stimulated fusion of v-SNARE vesicles, containing donor and acceptor FRET pairs, with unlabeled t-SNARE vesicles leads to an increase in FRET-donor fluorescence. Fusion assays were carried out with either GUVs or SUVs. (B) Representative fluorescence microscopy (left) and electron microscopy (right) images of vesicles used in this study. Scale bars: 8 μm (left) and 200 nm (right). (C) Absolute NBD de-quenching signals for C2A-C2B/C2A-C2BCLM-stimulated fusion of GUVs (left) and SUVs (right). Addition of detergent produced a maximum fluorescence signal for normalization. (D) Comparison of the % syt stimulated fusion for WT C2A-C2B and the tubulation deficient mutant, C2A-C2BCLM, for GUV (left) and SUV (right) fusion assays. Relative to WT, the extent of C2A-C2BCLM stimulated fusion shows a clear loss-of-function in the GUV system (left) as compared to the SUV system. 1 mM Ca2+ was added at t = 2 min to activate syt and trigger fusion. (E) Extent of stimulated fusion for GUV (left) and SUV (right) systems plotted for four syt constructs. (F) Left: GUV-GUV fusion was blocked by pretreatment with botulinum neurotoxin B or by the use of a truncated form of SNAP-25, which mimicked the cleavage product of botulinum neurotoxin E, or by addition of the cytoplasmic domain of synaptobrevin (cd-syb) or the cytoplasmic domain of the t-SNARE heterodimers (cd-dimer). Right An SDS-PAGE gel showing that botulinum neurotoxin B efficiently cleaved GUV embedded syb.