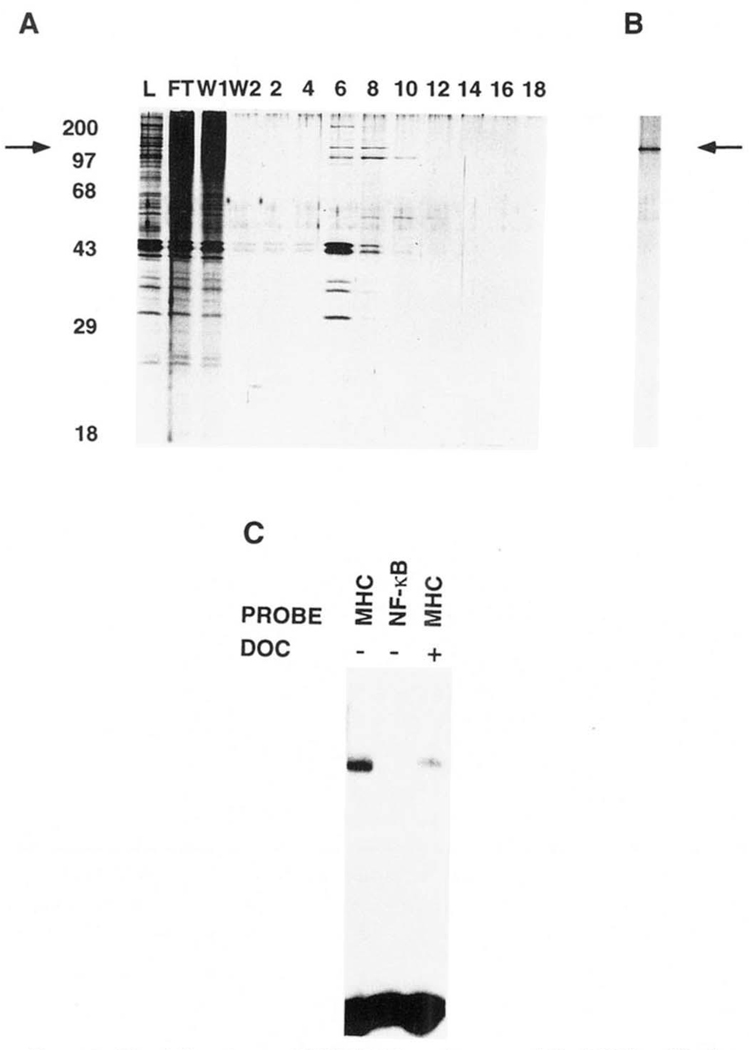
FIG. 2. Purification of H2TF1 by site-specific DNA affinity chromatography.
H2TF1-containing fractions eluted from the heparin-Sepharose column were pooled and desalted on Sephadex PD-10 columns to a final salt concentration of 50 mm KCl. Highly purified sonicated salmon sperm DNA was added to the desalted pool, to a final concentration of 500 mg/ml. The pool was then loaded on a 1.0-ml MHC κB site column, constructed as described under “Materials and Methods.” In some cases the loading was done more than once, but multiple loadings were later shown to be unnecessary for efficient binding (defined as >50% of loaded activity bound, data not shown). The loaded column was washed with 100 mm KCl and then developed with a 100–500 mm KCl gradient, all in buffer Y. The fractions containing H2TF1 activity were then pooled and desalted on Sephadex PD-10 gel filtration columns to a final salt concentration of 50 mm KCl. Sonicated salmon sperm DNA was again added to the pool, to a final concentration of 500 mg/ml. Chromatographic separation on the MHC κB site column was then repeated as above. A, SDS-PAGE of proteins eluted by a linear KCl gradient on the second round of MHC κB site affinity chromatography. The gel was run by the method of Laemmli (1970) and stained with silver by the method of Blum et al. (1987). Representative samples of loaded material (lane L), flow-through (lane FT),the first half of the wash (lane W1), the second half of the wash (lane W2),and fractions eluted by the linear KCl gradient from 190 to 580 mm KCl (lanes 2–18) were electrophoresed. Molecular weight standards (Mr) were myosin (200,000), phosphorylase b (97, 400), BSA (68,000), ovalbumin (43,000), carbonic anhydrase (29,000) and β-lactoglobulin (18,400). The arrow indicates the mobility of a 110,000 Mr polypeptide which further co-purified with H2TF1 activity and cross-linked specifically to the MHC κB site. B, SDS-PAGE of the fraction containing the peak H2TF1 activity, eluted from the third round of MHC κB site affinity chromatography. The protein species indicated by the arrow at 110,000 Mr co-eluted with the H2TF1 activity. The molecular weight standards for this gel were essentially the same as for A. C, gel shift assay of the fraction from the third affinity purification with peak H2TF1 activity, shown in B. The probes are as indicated. DOC +/− indicates the presence or absence of deoxycholate. The complex observed with the MHC WT probe was competed by a 40-fold excess of unlabeled MHC WT competitor, but not MHC MT, κB, or BSCRIPT (data not shown).