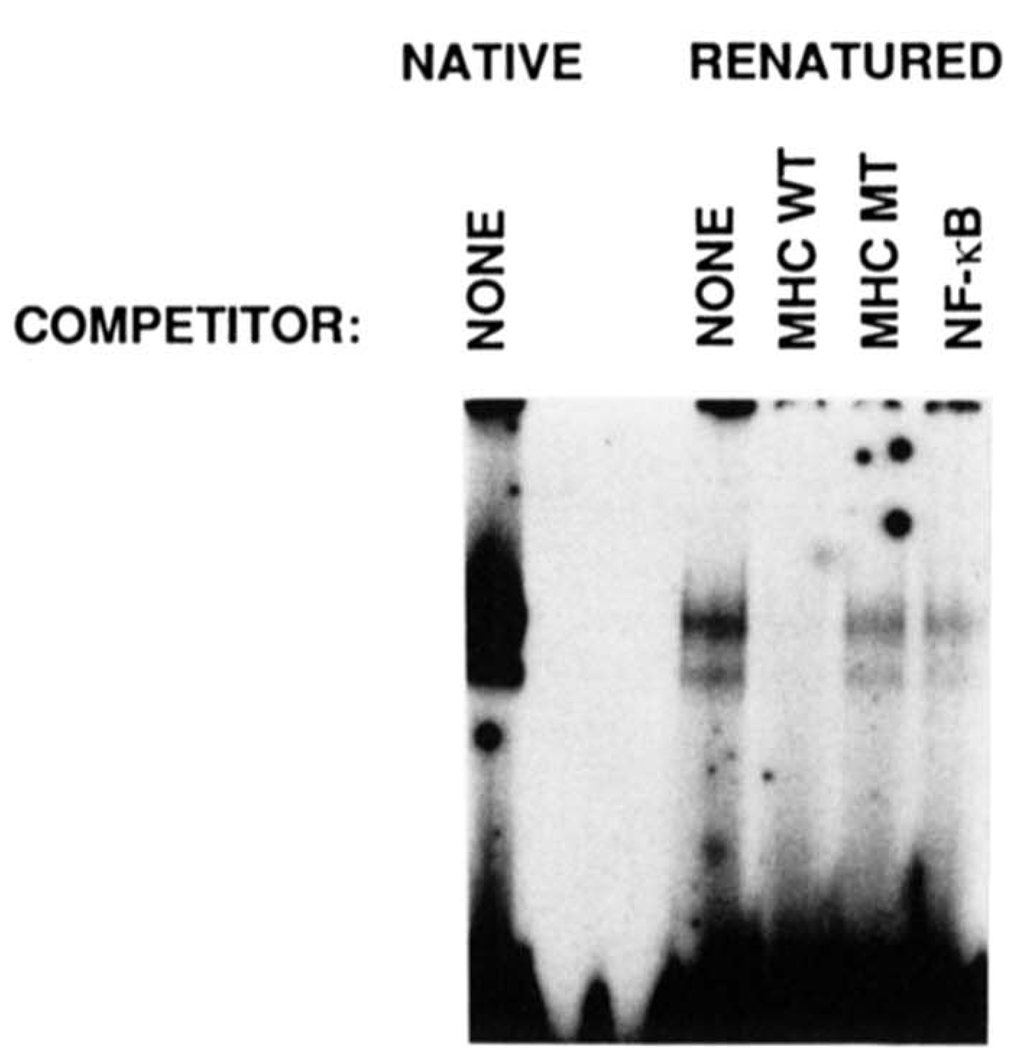
FIG. 4. Renaturation of H2TF1 sized by SDS-PAGE and demonstration of the DNA-binding specificity of the renatured protein.
H2TF1 was renatured from horizontal sections of an SDS-polyacrylamide gel according to a protocol modified from those of Baeuerle and Baltimore (1988) and Hager and Burgess (1980), described under “Materials and Methods.” Renatured H2TF1 was incubated with wild type MHC κB probe in the presence or absence of competitor DNA and assayed for DNA-binding by gel shift analysis. The mobility of the renatured protein. DNA complex (RENATURED) was identical to the starting material (NATIVE). Either wild type MHC κB site (MHC WT), double point mutant MHC κB site (MHC MT), NF-κB site(NF-κB), or no (None) competitor was added to the gel shift assays, and the result demonstrated that the renatured H2TF1 had the same DNA-binding specificity as the native material. The competitor DNA species were added at a 50-fold excess over the probe.