Abstract
Zebrafish have become a model organism in many areas of research and are now being used with more frequency in the classroom to teach important biological concepts. The two guided inquiry exercises in this article are each aimed at a different level of instruction, but each can be modified to fit the needs of many high school or college-level courses. The “Zebrafish Development and Environment” exercise teaches high school students about zebrafish development by presenting a series of embryos at different ages. Without access to visual references, students are asked to rank developing zebrafish by age and explain their choices. The students also learn about the heart and circulatory system and the effects of temperature on physiological processes. The second exercise, “Oxygen Consumption,” is a 2-week laboratory designed for introductory college biology majors and involves the concept of oxygen consumption as a predictor of metabolic rate. During the first week of lab, students are introduced to the concept and learn how to measure oxygen consumption in zebrafish. In the second week, they perform an instructor-approved experiment of their own design, analyze the results using statistics, and write a report.
Zebrafish Development and Environment—A High School Exercise
Background
This exercise was created in response to my daughter wanting me to bring zebrafish into her 10th-grade biology classroom. A simple show and tell with zebrafish eggs would have sufficed. However, the opportunity to scientifically engage potential career biologists could not be ignored. This exercise was designed to supplement their current discussions of mitosis and development. The challenge of this particular exercise was creating an effective guided inquiry lab that could be performed within the 50-min class time (which includes a short presentation and a question period).
Prior to the day of the exercise, the teacher was provided the outline to facilitate interaction and secure materials. The role of the high school teacher can range from one of support to completely running the exercise. However, guided inquiry exercises must have someone knowledgeable overseeing this exercise for them to be effective.
The focus of this exercise was on the effects of the environment on development, which is a major aspect of my own research. In early biology education, we are taught that animals are the products of genetic and environmental factors (“nature versus nurture”). In this exercise, students measure the effects of temperature on zebrafish heart rate in real time and think about how the environment could have a major impact on ectotherm development (Appendix 2). However, before they arrive at this phase, they must complete a brief exercise on zebrafish development. Without visual references, students in small groups examine various stages of zebrafish embryos and larvae and attempt to put them in order from youngest to oldest. Perhaps the most challenging aspect of this exercise is to communicate their rationale for their order.
By listening to a rationale for developmental order, I am able to gauge the students' understanding of developmental biology. The active process in which they are engaged is typically called constructivism. Students, even without formal instruction on developmental biology, are able to construct their own knowledge from linking past experiences with present information. This guided inquiry format emphasizes the student's ideas and allows them to form deeper understanding of important scientific concepts, all originating from a simple but well thought out series of experiments. While no data have been collected regarding the success of students in providing the correct developmental order, most of the groups either order them correctly or have one group out of place.
Overview
The introduction to this exercise entails a 10-min PowerPoint presentation based on my research. Obviously, this can be modified to fit the nature of the classroom visit and the background of individual researchers. Within this presentation, I feel that it is very important to convey what a biology professor does on a daily basis. Many students do not know the breadth of careers available to them if they are interested in biology, and even fewer really understand how science is actually done (and now they get to do it themselves).
The initial part of the exercise does require preparation many days in advance. Access to a breeding population of zebrafish or the ability to order various stages of zebrafish eggs is a prerequisite (Westerfield, 2000). I have outlined preferably five different ages of embryos/larvae. This seemed to be a very manageable number both in preparation and in execution. The oldest larvae were approximately 4 days old. The requirement for the oldest larvae is simply that they be old enough to have a beating heart, but not so old as to be free swimming. To reduce the number of breedings, clutches bred a day or two earlier were separated into two temperature groups: the standard 28.5°C incubating temperature and a colder 19°C–20°C room temperature so as to slow development. Finally, the morning of the exercise a new batch of fertilized eggs was obtained to show the earliest possible stages of development.
Following the introduction, the students will be broken into smaller groups, and each group will be given five containers each containing embryos/larvae. They are asked to view these under a dissecting scope and order the five groups from youngest to oldest, explaining what characteristics led them to their outcome. The students are then asked to locate an individual cell in any of the age groups and explain which groups have easier cells to locate. The goal of this question is to lead students to discover and/or reinforce the developmental process of cleavage—cells in younger embryos are larger and thus easier to identify. In the third section, the students are asked to find external movement in the age groups. This allows them to think about muscles, the opercula, and fin movement and why these various movements might be necessary, even while still inside the egg. Finally, the students are asked to identify internal movement. This should allow them to discover the beating heart and circulation of blood. After obtaining ice and chilling the container, they will notice the heart slow down and will have to think about why cooling slows a physiological process.
Perhaps the largest variation in the procedure was the overall timing of the various sections within this exercise. It is not possible, in many cases, for students to complete the entire exercise according to the schedule I have outlined in Appendix 1. Students often spend more time than expected just observing and discussing the various aspects of developing zebrafish under the dissecting scope. This is such an important aspect of the scientific process that I often forgo the process of cooling the embryos. Therefore, if time is flexible, I recommend more than 50 min, perhaps 90 min if retaining the presentations and question period.
The student response to this exercise has been overwhelmingly positive. This is attributed primarily to students being able to actually view the beating heart and circulation in real time. Unfortunately, even in well-endowed high school biology laboratories, the chance of students learning from a living vertebrate and viewing a physiological event is very rare. In fact, with most schools on a strict budget, textbook and paper learning typically replaces the quality laboratory experience. The following are two actual student comments that I will never forget:
“I learned more today than I have all year.”
“If biology was like this all the time I would be a biologist.”
While positive for me, this does not often speak well for the state of science education in our high schools. However, I use this to fuel my ambitions to design new exercises for the classroom that may inspire a few future biologists.
Oxygen Consumption and a Predictor of Metabolic Rate
Background
This laboratory exercise is one of 10 two-week modules that were created for the introductory biology course at the University of Akron as a direct result of an NSF grant to Dr. Richard Londraville and Dr. Peter Niewiarowski. This grant, titled “Connecting Research and Teaching with Inquiry-Based Biology Laboratories,” focused on changing the entire first year biology major experience from cookbook labs to engaging guided inquiry learning exercises. Another feature of these new labs was the focus on actual faculty research within our department. This gives biology students early exposure to what professors in the department are actually doing. I was asked to contribute an exercise to this series, and with the help of Jeff Spencer (Anatomy and Physiology Lab Coordinator) and Ashley Ramer (Principles of Biology Lab Coordinator), we designed a 2-week lab exercise (lab sessions are 3 h) that focused on the aspect of oxygen consumption as a predictor of metabolic rate (Appendices 3–7). Through this exercise, students learn how oxygen consumption relates to metabolic rate, how to measure oxygen consumption in an ectotherm, and how to design their own experiment within a set framework.
Overview
This exercise focuses on the measurement of oxygen consumption. Therefore, the minimal investment for this exercise would be a basic oxygen meter that measures dissolved oxygen in water. We are fortunate to have many of these, and they are connected to a data collection system (LoggerPro; Vernier Software & Technology, Beaverton, OR). Running dissolved oxygen measurements on a computer system has the advantage of allowing the students to understand the concept of calibration and electrode stabilization more so than on a hand-held meter. We recommend one experimental setup per four students, but realize this will be limited by budget. Using a hand-held dissolved oxygen meter (obtained at any high end aquatics store) is the most cost-effective way to run these experiments; however, the students will have to manually record the readouts over time.
The exercise in week 1 is very straightforward (Appendices 3 and 4). This allows the student in groups of four to learn the technique, understand the calculations, and acclimate to live animal experiments. A zebrafish is obtained from the laboratory breeding stocks and placed quickly in a 500 mL Erlenmeyer flask filled with dechlorinated room temperature (∼21°C) tap water. The flask is then capped with a rubber bung containing the oxygen probe. Following a 5-min acclimation period, the computer software begins to record the oxygen concentration in the water. In a functional setup, the oxygen concentration should drop significantly over the 30-min measurement time. The students are then asked to measure the actual volume of water in the flask as well as the mass of the zebrafish so that they can then calculate oxygen consumption.
In week 2, each group of four students, perhaps for the first time, has to implement their own experiment (designed during week 1) manipulating oxygen consumption of zebrafish (Appendix 5). Although the approved animal care protocol gives very wide latitude, the teaching assistants, the laboratory coordinator, and I spend careful time evaluating all of the proposed experiments to ensure that the animals are not unduly stressed. The most common examples of environmental manipulations are temperature, salinity, oxygen level, pH, light, and activity level. Students have also looked at sex and size differences in oxygen consumption. In the data analysis portion of the exercise, students learn the basics of statistical analysis and will prepare a report based on their collected data (Appendix 6). The students with the best projects will be asked to create a poster presentation for the undergraduate research competition held every spring. Finally, questions related to faculty zebrafish research challenge the students to think about oxygen consumption in a relative manner based on body mass (Appendix 7). This aspect by far has been the most challenging for students, but also allows exceptional students to flourish.
As you read through Appendices 3 and 4, it is very obvious that this exercise need not be centered on zebrafish. Any small aquatic ectotherm will suffice. However, the opportunity here to introduce students to perhaps one of the most influential model organisms in the past decade is difficult to ignore. The disadvantage of using vertebrates is, of course, the requisite animal care and use application that must be approved prior to execution. We felt that the exercise was important enough to proceed with this extra step. If a substitution were made, any aquatic invertebrate, such as a small crayfish or snail, will suffice and would not require any special protocol approvals. When choosing a crayfish or other similarly sized aquatic ectotherm, adjustments may need to be made to the Erlenmeyer flask size depending on the size of the organism. The goal is for the organism to use approximately 20% of the oxygen in the flask over 30 min.
This exercise could also be modified for use in an advanced high school biology course. It is also an excellent opportunity for high school teachers to team up with and be trained by researchers to provide an advanced guided inquiry experiment to future university students. In many cases, these partnerships are paramount in making arguments to high school administration for biology laboratory equipment funding.
A surprising consequence of relating laboratory exercises to faculty research early in an undergraduate career is the increase in student interest of department research. I have experienced a significant increase in undergraduate research in my lab, and because these students are following up on this interest at an earlier point in their career, they are remaining in my lab for longer periods. The result is an increase in not only the quantity, but also the quality of data collected in my lab.
Appendix 1. High School Experiment Outline Provided to High School Teacher
Zebrafish Development and Environment—Teacher Information
Materials needed in classroom/lab:
Microscopes/dissecting scopes
Thermometers
Sink with running hot and cold water
Ice
Data projector (to hook up to a computer for brief presentation on zebrafish development)
Materials brought from university:
About 50 zebrafish eggs/embryos/larvae in each of 5 different age groups
Eye droppers
Small containers to sort eggs/embryos/larvae
Cooler for transport
Rough Time Outline:
Introduction (15 min)
Distribute and Begin Lab Exercise (5 min)
Completion of Lab Exercise (20 min)
Brief Question Period Followed by Student Questions (10 min)
Exercise Outline:
Introduction
1) I would like to briefly explain what my job entails and what type of education is needed to become a professor of biology. I feel this is important because many students with families who are not in academia assume the only pathways for those who love science are medical doctors or engineers.
2) With a brief PowerPoint presentation, I would like to get them thinking about mitosis, but in a broader context of development. I will ask them questions leading them to think about endothermic development and the concept of “nature versus nurture.” I will then move into ectothermic development and specifically fish development. What is the “nurture” component? How does it affect mitosis?
3) This will lead into the instructions for a brief exercise using zebrafish embryos.
Lab Exercise
1) The student groups will be given at least 5 groups of zebrafish embryos/larvae and they will be asked to observe them and record their observations.
2) They will then (depending on time) heat or cool the container containing the eggs and record their observations (see Appendix 2).
3) The goal will be for the students to notice that temperature has a significant effect on zebrafish heart rate.
Question Period
1) I would like them to participate in answering the questions I pose to the class, most of which will be written on their instruction sheet. I hope to guide them into thinking about the environment (temperature, oxygen level, etc.) as major factors affecting developmental rate and success in ectotherms.
2) Finally, time permitting, I would enjoy answering any questions from the class!
Appendix 2. High School Experiment Worksheet Provided to Students
Zebrafish Development and Environment—Student Exercise
In front of you are different stages of zebrafish. Follow the instructions below and try to answer the questions.
1) Obtain an embryo or larvae from each container (one at a time) with an eyedropper and observe it under the scope. Order the groups from youngest to oldest. Explain what characteristics helped you to determine the age of the fish.
2) Try to locate an individual cell. Which group has the easiest cells to locate? Why do you think that is?
3) Is there a group that has external movement? Why do you think this is?
4) Is there a group that has internal movement? What is this movement called? What happens to the movement when the container is chilled? Why do you think this happens?
Appendix 3. Undergraduate Experimental Outline and Background Provided to Students in Week 1
Oxygen Consumption (As a Predictor of Metabolic Rate)
BASED ON THE RESEARCH OF Dr. BRIAN BAGATTO
Authors – J. Spencer, A. Ramer, and B. Bagatto
Lab Outline
Lab One
TAKE A PRELAB QUIZ (COVERS INFORMATION FROM PAGES 2–3)
MEASURE THE OXYGEN CONSUMPTION OF ZEBRAFISH
ANALYZE DATA
DEVISE AN EXPERIMENT TO ALTER METABOLIC RATE
WHAT YOU HAND IN FOR LAB ONE
QUIZ
EXPERIMENTAL DESIGN
Lab Two
COMPLETE YOUR EXPERIMENT
STATISTICALLY ANALYZE YOUR RESULTS
REVIEW FACULTY DATA WITH GROUP MEMBERS
WHAT YOU HAND IN FOR LAB TWO
INTERPRETATION OF DR. BAGATTO'S DATA
RESULTS OF EXPERIMENT
Points
Lab One
PRELAB QUIZ (INDIVIDUAL) 10 POINTS
OXYGEN CONSUMPTION DATA (GROUP) 5 POINTS
PROPOSAL WORKSHEET (GROUP) 20 POINTS
Lab Two
EXPERIMENT RESULTS (GROUP) 20 POINTS
FACULTY DATA INTERPRETATION (INDIVIDUAL) 10 POINTS
Ectotherm Metabolism—Background
Animal metabolism is a continuous process that encompasses all reactions in the body. Nutrients are absorbed from the digestive tract and serve as fuel for catabolism. The macromolecules (carbohydrates, proteins, lipids, and nucleic acids) are broken down into three-carbon molecules (pyruvate) which enter the mitochondria. In the presence of oxygen, pyruvate fuels ATP synthesis utilizing the Kreb's cycle, electron transport chain, and chemiosmosis. In this process, oxygen is used and carbon dioxide is produced. The rate of oxygen consumption can therefore indicate an animal's metabolic rate.
Metabolic rate is directly linked to the temperature of an animal. An ectotherm (outdated term = cold-blooded) must gain heat from the surrounding environment since their own metabolism is not sufficient to heat the animal internally. Endotherms (old term = warm-blooded), in contrast, derive most or all of their body heat from their own metabolism. Other factors that may alter an aquatic ectothermic organism's metabolic rate include activity levels, pH, salinity, ions, size, light, movement, etc.
Zebrafish are ectothermic organisms that possess gills for acquiring oxygen from the water. Dissolved oxygen (DO) refers to the volume of oxygen that is contained in water. Oxygen enters the water by photosynthesis of aquatic biota and by the diffusion of oxygen across the air–water interface. The amount of oxygen that can be held by the water depends on the water temperature, salinity, pressure, and agitation.
Classification of Zebrafish:
PHYLUM: Chordata
CLASS: Actinopterygii
ORDER: Cypriniformes
FAMILY: Cyprinidae
GENUS: Danio
SPECIES: D. rerio
SCIENTIFIC (BINOMIAL) NAME: Danio rerio
Appendix 4. Undergraduate Experimental Protocol Provided to Students in Week 1
Lab Part One:
The metabolic rate of the zebrafish can be measured by the following steps:
1. Fill the Erlenmeyer flask with water from the plastic carboy.
2. Carefully and quickly use a net to obtain one zebrafish and transfer it to the flask.
3. Cap the flask with the attached oxygen probe. Be sure that water overflows from the flask (this ensures that there are not any air bubbles present). If water does not overflow, fill it up and try again. Be sure this is done away from electrical outlets and equipment!
4. Let the zebrafish remain undisturbed for 5 minutes.
5. Record the sex of the fish.
6. In LoggerPro, click on the clock icon (next to the “Collect” button). Change the length to 30 minutes and record 60 samples/minute.
7. Now hit “Collect” and begin to record dissolved oxygen (DO) values. Watch the values, and once they have stabilized (<30 seconds), click “Stop.”
8. Hit “Collect” again, and if it asks, select “Erase and Continue” to begin to record your data.
9. Once oxygen sampling is finished click “analyze” and then “linear fit.” This draws the best straight line through your data. The equation it gives you is for that line (y = mx + b), where y is dissolved oxygen, m is the slope, x is time, and b is the point where the line crosses the y-axis. “Cor” is the correlation coefficient—the closer you are to “1”—the better.
10. Print your graph (Hit Shift + Print Screen, open up Word, and hit Edit, Paste).
11. Remove the DO probe and record the final water temperature.
12. You now need to measure the amount of water in the flask and the weight of the zebrafish. First, obtain a small beaker filled halfway with water and tare this on the balance. Pour the water from the flask over a net positioned above a graduated cylinder (try to prevent any water from spilling out of the flask). Once the fish is caught in the net, quickly transfer it to the tared container. Record the volume of water in the graduated cylinder (in mL) and the weight of the fish (in grams).
13. Obtain fresh water and repeat steps 3–12 if time permits.
14. Return the zebrafish to its original housing container.
➣ Record your data for the following items:
1. Sex of your zebrafish
2. Weight of your zebrafish (in grams)
3. Initial oxygen within closed system (calculated value)
4. Temperature when experiment began
5. Final oxygen within closed system (calculated value)
6. Temperature after experiment
7. Oxygen consumed per minute
8. Oxygen consumption per minute/gram tissue
Record your group's data onto the Excel spreadsheet that's projected at the front of the room (5 points).
To Analyze Data and Obtain Calculated Values—An Example:
Suppose the flask has 233 mL of water (the amount in the graduated cylinder = total volume – zebrafish and submersed portion of oxygen probe). Let's say that the water contains 7.2 mg/L oxygen at the beginning of the experiment. So, in the closed system there is 1.68 mg of oxygen to begin the experiment (0.233 L×7.2 mg/L = 1.68 mg oxygen).
After an hour the oxygen concentration is 4.1 mg/L. There is 0.96 mg of oxygen (0.233 L×4.1mg/L = 0.96 mg oxygen). 1.68 – 0.96 = 0.72 mg of oxygen has been consumed in an hour.
0.72 mg oxygen/60 minutes = 0.012 mg oxygen per minute; suppose the zebrafish weighs 0.5 grams = 0.012/0.5 = 0.024 mg oxygen/minute/gram tissue
Appendix 5. Undergraduate Experimental Design Sheet Provided to Students in Week 1
Design an Experiment Lab One and Conduct the Experiment Lab Two
Design an experiment in which you will manipulate a zebrafish's normal metabolic rate (as measured in lab one) or one in which you will compare the metabolic rate of different zebrafish. Outline this experiment on the proposal worksheet below. If you decide to manipulate the zebrafish's environment (temperature, pH, ions), you must have the experimental apparatus in equilibrium for at least 15 minutes before you can place the zebrafish in the flask.
Proposal of Experimental Design (20 points)
Name ___________________________________________
Name ___________________________________________
Name ___________________________________________
Name ___________________________________________
1. State your hypothesis and the motivation for your hypothesis.
2. How do you plan on conducting your experiment (be specific!)?
3. Describe, in detail, how you will analyze your data (which statistical test will you use?).
4. What is your sample size?
5. Sketch a graph to show how you will present your data.
Appendix 6. Undergraduate Experimental Protocol Provided to Students in Week 2
Lab Two Procedures:
1. Complete your experiment and record your raw data.
2. Use the appropriate statistical test to analyze your data (t-test, chi-square test, or regression analysis).
-
3. Prepare a typed summary (20 points total).
Include in your summary:
a. your hypothesis (2 points)
b. motivation for your hypothesis (rationale for your experiment) (1 point)
-
c. description of your methods
i. description of the groups (control, if applicable, and how treatment differs) (2 points)
ii. description of how you actually conducted your experiment (in detail!) (2 points)
iii. statement of sample size (1 point)
iv. discuss which statistical test you used (1 point)
-
d. results
i. graph your data (column for t-test or scatter for regression analysis) (2 points)
ii. include raw data and Excel output summary sheet (1 point)
iii. briefly state the results of your statistical test (p-value) (2 points)
-
e. interpret statistics and discuss whether your hypothesis was supported or not (2 points)
i. if not supported, discuss why this may be (alternate explanations, design limitations) (1 point)
f. discuss what you would do next if you had time (3 points)
4. Staple and turn in your raw data, graph (if applicable), statistical analysis, and summary.
Appendix 7. Undergraduate Data Interpretation Exercise Provided to Students in Week 2
Faculty Data Interpretation
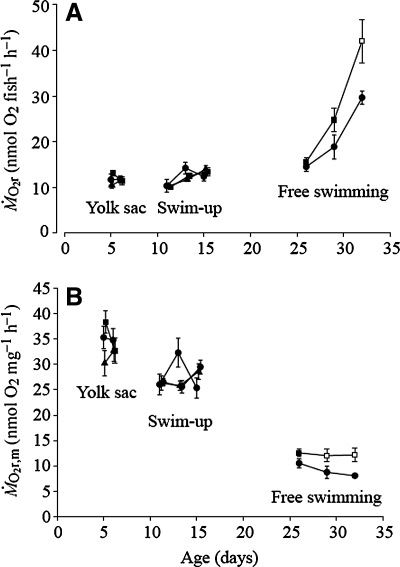
The data displayed below was taken from a study conducted by Dr. Brian Bagatto et al. (Fig. 1).1 The goal of the research was to determine if swim training has any effect on larval zebrafish oxygen consumption. The study subjects for this experiment included groups of individuals that were either 4 days old (called yolk-sac larvae), 9 days old (called swim-up larvae), or 21 days old (called free swimming larvae). Individuals within each age group were further divided into either a control group or one of two treatment groups. The study subjects within the treatment groups were swim trained for 48 hours within tubes with water velocities of either 2 body lengths per second (2BL s−1) or 5 body lengths per second (5BL s−1).
FIG. 1.
Routine oxygen consumption (MO2r) (A) and mass-specific routine oxygen consumption (MO2r,m) (B) of zebrafish yolk-sac larvae (day 4), swim-up larvae (day 9), and free-swimming larvae (day 21), exposed to training. Circles, control group; triangles, training at 2 BL s−1; squares, larvae training at 5 BL s−1. An open symbol indicates a value significantly different from the corresponding control value. Values are means±SEM; n = 8 for each group. (Figure from Bagatto et al.1)
Treatment subjects were removed from the swim training tubes and each individual was placed into an apparatus used to measure routine oxygen consumption (= oxygen consumed at rest). Control subjects were also placed into apparatus and their routine oxygen consumption measured.
Graph Interpretation
Circle = control group
Triangle = Training at 2BL s−1
Square = Training at 5BL s−1
Open Symbol = Value significantly different from corresponding control value
Real-World Scenario That May Help You Answer the Questions Below:
Boxers are divided into various weight classes that span from minimumweight, also called mini flyweight or straw-weight (<105 pounds), to heavyweight (>200 pounds). It is highly unlikely that a champion from within the minimum-weight class would win if matched against a heavyweight. However, when analysts try to determine the best-of-best in the sport, they compare boxers across all classes on a “pound-for-pound” basis.
1. Which panel in Fig. 1, A or B, is a “pound-for-pound” comparison of zebrafish oxygen consumption (focus on the y-axis)?
2. How is a “pound-for-pound” measurement obtained (think back to your data and how you determined this)?
3. Why display both overall oxygen consumption data and “pound-for-pound” data (what's the purpose)?
-
4. In panel A, which group of larvae has the overall highest routine oxygen consumption?
a. Yolk sac
b. Swim-up
c. Free swimming
-
5. You compare a milligram of tissue from a large larva with a milligram of tissue from a small larva.
Which would have lower mass-specific routine oxygen consumption?
i. Smaller larvae have lower per mg oxygen consumption.
ii. Larger larvae have lower per mg oxygen consumption.
-
6. Does swim training have any significant effect on routine oxygen consumption within any age category?
*If yes, explain which age group(s) and which form of treatment (2 or 5BL s−1)
7. Why might swim training have this effect on zebrafish larvae (think at the subcellular level)?
Literature Cited: http://en.wikipedia.org/wiki/Boxing_weight_classes
Acknowledgments
The author would like to thank Ashley Ramer and Jeff Spencer for their significant contributions on the undergraduate protocol. The design and implementation of this protocol would not have been initiated without an NSF DUE, CCLI grant awarded to Dr. R. Londraville and Dr. P. Niewiarowski.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bagatto B. Pelster B. Burggren WW. Growth and metabolism of larval zebrafish: effects of swim training. J Exp Biol. 2001;204:4335–4343. doi: 10.1242/jeb.204.24.4335. [DOI] [PubMed] [Google Scholar]
- 2.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene, OR: University of Oregon Press; 2000. [Google Scholar]