Table 2.
Palladium-Catalyzed Carboamination of N-Protected γ—Aminoalkenes with Functionalized Aryl Bromidesa
| entry | amine | aryl bromide | product | dr | yieldb |
|---|---|---|---|---|---|
| 1 |
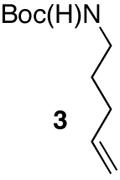
|
|
|
75 | |
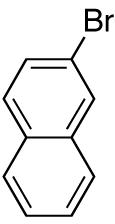
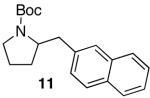
| 2 |
|
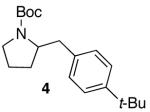
|
82 | ||
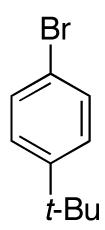
| 3 |
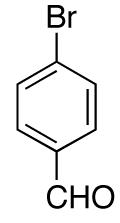
|
|
78e | ||
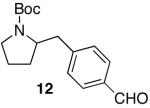
| 4 |
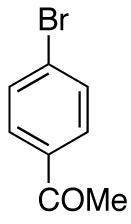
|
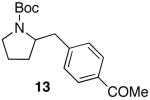
|
76e | ||
| 5 |
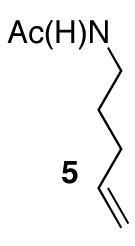
|
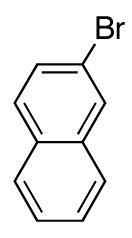
|
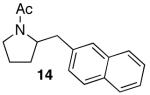
|
79 | |
| 6 |
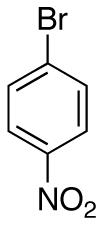
|
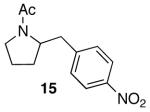
|
76c,e | ||
| 7 |
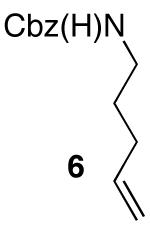
|
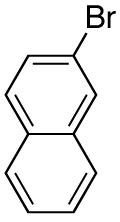
|
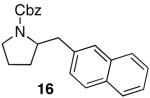
|
88 17d |
|
| 8 |
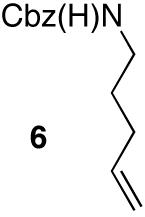
|
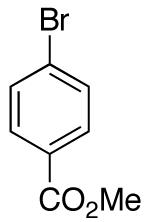
|
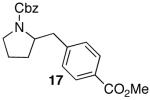
|
— | 88e |
| 9 |
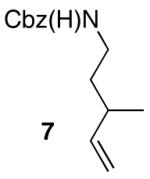
|
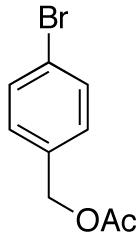
|
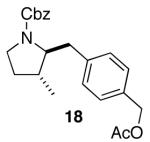
|
12:1 | 80 |
| 10 |
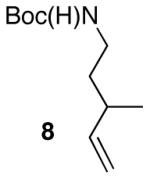
|
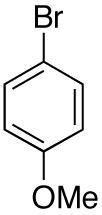
|
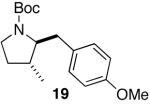
|
15:1 | 76 |
| 11 |
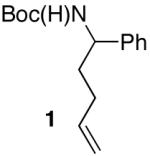
|
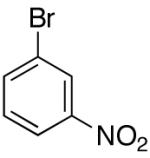
|
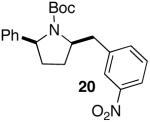
|
>20:1 | 75 |
| 12 |
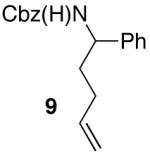
|
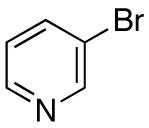
|
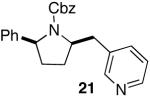
|
>20:1 | 74 |
| 13 |
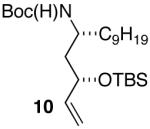
|
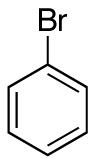
|
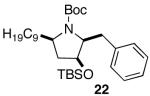
|
>20:1 | 71 |
| 14 |
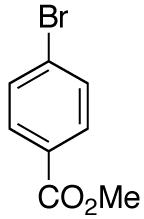
|
|
>20:1 | 73 |
Conditions: 1.0 equiv amine, 1.2 equiv ArBr, 2.3 equiv Cs2CO3, 2 mol % Pd(OAc)2, 4 mol % Dpe-phos, dioxane (0.2–0.25 M), 100 °C.
Yield refers to average isolated yield obtained in two or more experiments.
Dppe used in place of Dpe-phos.
NaOtBu used in place of Cs2CO3.
The reaction wasconducted at 85 °C in DME solvent.
