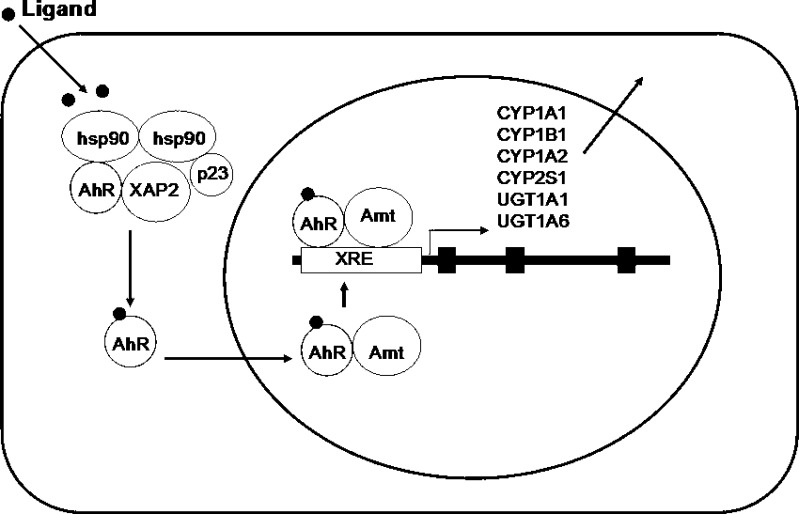
Fig. 3.
A schematic illustration of the aromatic hydrocarbon receptor (AhR)-mediated induction of phase I and phase II drug metabolizing enzymes and drug transporters such as human CYP1A1, 1B1, 1A2, and 2S1, UGT1A1 and 1A6, and MDR1/ABCB1. The AhR exists as cytoplasmic aggregates bound to two 90-kDa heat-shock proteins (Hsps), the cochaperone p23 and a 43-kDa immunophilin-like protein hepatitis B virus X-associated protein 2 (XAP2). Upon binding a ligand, after the replacement of its associated molecule with AhR nuclear translocator (Arnt) to form a heterodimer with release of 90-kDa heat-shock proteins, AhR translocates into the nucleus. This heterodimer interacts with a 5′-GCGTG-3′ DNA sequence, the core binding motif of the xenobiotic response element (XRE) or dioxin response element of the target genes encoding CYP1A1, 1B1, 1A2, and 2S1, and uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) and UGT1A6