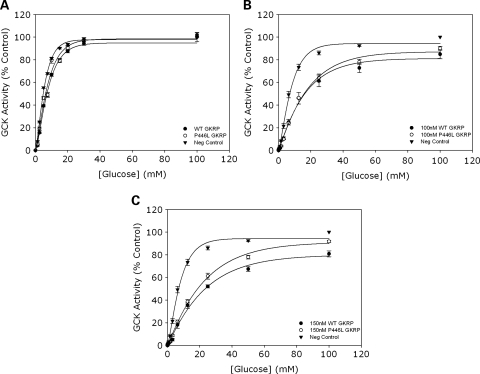
Figure 1.
(A) Competitive inhibition of GCK using equivalent activities of WT and P446L-GKRP (n = 24). Competitive inhibition of 10 m U/ml GCK by one GKRP unit (WT=black circles, P446L=white circles, negative control=black triangles) was observed over a glucose concentration range of 0–100 mm. Each data point was plotted as a percentage of the negative control in which GKRP was absent and glucose concentration equaled 100 mm. Statistical analysis revealed no difference between the intrinsic inhibitory capacity of WT and P446L-GKRP. (B and C) Competitive inhibition of GCK using equimolar concentrations of WT and P446L-GKRP (n = 3). GCK activity was assayed in the presence of 0, 100 and 150 nm WT (black circles) and P446L (white circles) GKRP, over a glucose concentration range of 0–100 mm. Each data point was plotted as a percentage of the negative control in which GKRP was absent (black triangles) and glucose concentration equaled 100 mm. At 100 nm GKRP, there was statistically no difference between the responses of the two regulatory proteins (although it was noted that the variance of this data was larger than seen for other experiments and n = 3). At 150 nm GKRP, statistical analysis showed the intrinsic inhibitory capacity of P446L-GKRP to be significantly lower than that of the WT regulatory protein at [glucose] over 25 mm (P ≤ 0.03).