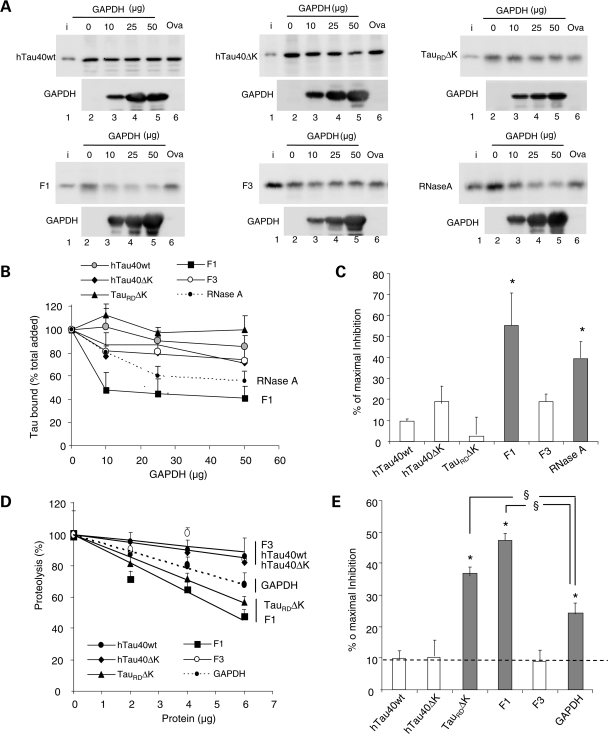
Figure 6.
Competition of the lysosomal association of different forms of Tau by substrates of CMA. (A) Effects of CMA substrate (GAPDH) and CMA non-substrate (ovalbumin) on binding and uptake of Tau by lysosomes. The different forms of Tau proteins (2 µg) and the indicated increasing concentrations of GAPDH or ovalbumin (Oval) were incubated with intact isolated lysosomes previously treated with protease inhibitors. Lane 1 shows 1/10 of the input (i) and insets show the amount of GAPDH associated with the lysosomal membranes. Only association of the F1 fragment and of RNase A (shown here as a positive control) could be competed by increasing concentrations of GAPDH, whereas ovalbumin did not modify lysosomal binding. (B) The percentage of the different Tau proteins and of RNase A bound to the lysosomal membrane in the presence of increasing concentrations of GAPDH was quantified in four experiments similar to the ones shown in (A). Values are mean ± SEM. (C) The maximal inhibition on Tau binding attained by GAPDH was calculated in four experiments similar to the ones shown in (A). Values are mean ± SEM (*P < 0.05). (D and E) Effect of different Tau proteins or GAPDH on the degradation of a pool of CMA substrates by intact lysosomes. Values expressed as % of proteolysis in the absence of Tau (D) or % of maximal inhibition of the degradation of the pool of proteins (E). Values are mean ± SEM of two experiments with triplicate samples. (*P < 0.05 compared with wild-type full-size Tau and §P < 0.05 compared with the inhibitory effect of GAPDH).