Abstract
Cyclin-dependent kinase 5 (cdk5) is a ubiquitous protein activated by specific activators, p35 and p39. Cdk5 regulates neuronal migration, differentiation, axonogenesis, synaptic transmission and apoptosis. However, its role in motor neuron development remains unexplored. Here, using gain and loss-of-function analyses in developing zebrafish embryos, we report that cdk5 plays a critical role in spinal and cranial motor neuron development. Cdk5 knockdown results in supernumerary spinal and cranial motor neurons. While a dominant negative, kinase-dead cdk5 promotes the generation of supernumerary motor neurons; over-expression of cdk5 suppresses motor neuron development. Thus, modulating cdk5 activity seems promising in inducing motor neuron development in vivo.
Keywords: motor neuron, neurogenesis, protein kinase, morpholino knockdown, zebrafish
Introduction
The central nervous system contains a large number of different cell types and has provided an avenue to study progenitor cell commitment and differentiation. Neural development of vertebrates is regulated by a precise coordination of cell proliferation and differentiation. Neural precursor cells are generated through a series of steps comprising neural induction, patterning, and neurogenesis [1, 2].
Cyclin-dependent kinase 5 (cdk5) was initially identified as a member of the cyclindependent kinase family [3] and is found in mitotic cells [4]. Cdk5 kinase activity is not stimulated by an associated cyclin but by its specific activators, p35 and p39 [5–7]. Cdk5 knockout mice exhibit defects in organization of the cortex and cerebellum and are embryonically lethal [8]. Over-expresion of cdk5 and p35 promotes neurite outgrowth of cultured cortical neurons whereas a dominant-negative mutant of cdk5 inhibits neurite outgrowth [9].
Cdk5’s role during early neural development is not known. In our earlier studies, we used injection of cdk5 siRNA to knockdown cdk5 expression in the embryos. In the siRNA-mediated cdk5 knockdown embryos, there was a marked decrease in the primary sensory neurons in the central and peripheral nervous systems, such as, the Rohon-Beard (RB) and trigeminal ganglion neurons [10–12]. Here, we show that inhibition of cdk5 protein expression by using specific antisense morpholino oligonucleotide (MO) dramatically induces motor neuron development. Conversely, cdk5 over-expression by mRNA injection suppresses motor neuron generation. Over-expression of a kinase-dead cdk5 mutant (cdk5 DN) induces motor neuron generation. These studies show, for the first time, that cdk5 can modulate motor neuron development.
Materials and methods
Animals
Zebrafish (Danio rerio) were kept and raised essentially according to standard procedures (NCI, NIH facility). Transgenic zebrafish that express green fluorescent protein (GFP) in the motor neurons were used in this study [13]. In these transgenic fish, GFP expression is driven by islet-1 promoter/enhancer sequences that were sufficient for neural-specific expression. The expression of GFP by the motor neurons in the transgenic fish enables visualization of the motor neurons, main axons, and the peripheral branches within the muscles.
Morpholino (MO) microinjection
Anti-sense oligonucleotide (MO) against zebrafish cdk5 coding sequence was designed and purchased from Gene Tools, LLC (Oregon, USA). The sequence of the translation-inhibitory morpholino for cdk5 is TCCAGCTTCTCATACTTTTGCATGG.
The morpholino (MO) was dissolved in Danieu’s buffer [14] before injection. MO was injected into each egg at one- to two-cell stage (10 ng/embryo). In vitro transcribed mRNA obtained from the zebrafish cdk5 cDNA cloned into the ClaI /Xba I sites of the PCS2 vector using the SP6 RNA polymerase (Ambion Inc., Austin, TX). The plasmid was linearized by Not I for in vitro mRNA transcription. Human inactive mutant cdk5 (K33T) in pcDNA3 (cdk5 DN) was a gift from Dr Li-Huei Tsai. In vitro transcribed mRNA of the kinase-dead human cdk5DN was obtained by T7 RNA polymerase mediated transcription of Stu I-linearized plasmid.
Western Blotting
Extracts were prepared from embryos microinjected with either control or cdk5 siRNA. Fifty micrograms of total protein were separated by 4–20% SDS-PAGE and immunoblotted to PVDF membrane. Cdk5 was detected using an antibody against mammalian cdk5. The immunoblots were developed for signal visualization by enhanced chemiluminescence (ECL) (Amersham, Chicago, IL).
Antibodies and chemicals
Rabbit polyclonal antibodies against mammalian cdk5 (C-8) and actin were purchased from Santa Cruz Biotech (Santa Cruz, CA).
Cdk5 activity assay
Embryos were collected at specified time points and were homogenized in 120 µl of lysis buffer (10 mM Tris-HCl, pH 7.5, 1% sodium deoxycholate, 1% Nonidet P-40, 150 mM NaCl, protease and phosphatase inhibitors). Homogenates were then sonicated and centrifuged for 5 min at 14,000 × g. Immunoprecipitations of extracts containing 200 µg of total protein were performed by adding 10 µl of the anti-cdk5 antibody (C-8) and incubating overnight at 4° C with constant rotation. Kinase activity assays were performed as described earlier [15].
Results
Cdk5 morpholino oligonucleotide (MO) and the human kinase-dead dominant negative cdk5 mutant (cdk5 DN) independently knock down cdk5 activity in zebrafish embryos
To investigate cdk5 function in motor neuron development in vivo, we performed loss of function analysis using morpholino antisense technology and gain of function analysis using capped RNA injections in a transgenic zebrafish line that drives islet-1 promoter green fluorescent protein (GFP) Tg (islet1: GFP) expression specifically in the motor neurons [13]. We determined MO efficacy by immunoblots for cdk5 protein in 26 hours post fertilization (hpf) cdk5 MO-injected embryo lysates and compared to the uninjected embryos and observed an ∼ 80% loss (Fig. 1 A , B) of the cdk5 protein in the cdk5 MO-injected embryos (Fig. 1 A, upper panel) in comparison to uninjected embryos along with no change in β-actin levels (Fig. 1 A, lower panel). Cdk5 activity was also drastically reduced in the cdk5 MO-injected embryos (Fig. 1 C, cdk5 MO lane). For GOF analysis, we injected 50 pg of zebrafish cdk5 or a kinase-dead human cdk5 (cdk5 DN) capped mRNA, and observed that in the zebrafish cdk5 mRNA-injected embryos, cdk5 activity increased significantly (Fig. 1 C, Cdk5 mRNA lane), while in the human cdk5 DN-injected embryos, the activity was reduced (Fig. 1 C, Cdk5 DN mRNA lane). Coomassie Blue staining indicates histone H1 substrate (Fig 1C, lower panel) and phosphorylated histone H1 levels are shown (cpm) to demonstrate the quantification of cdk5 activity among the experimental groups (Fig. 1 D).
Figure 1. Knockdown and over-expression of cdk5 activity in zebrafish embryos.
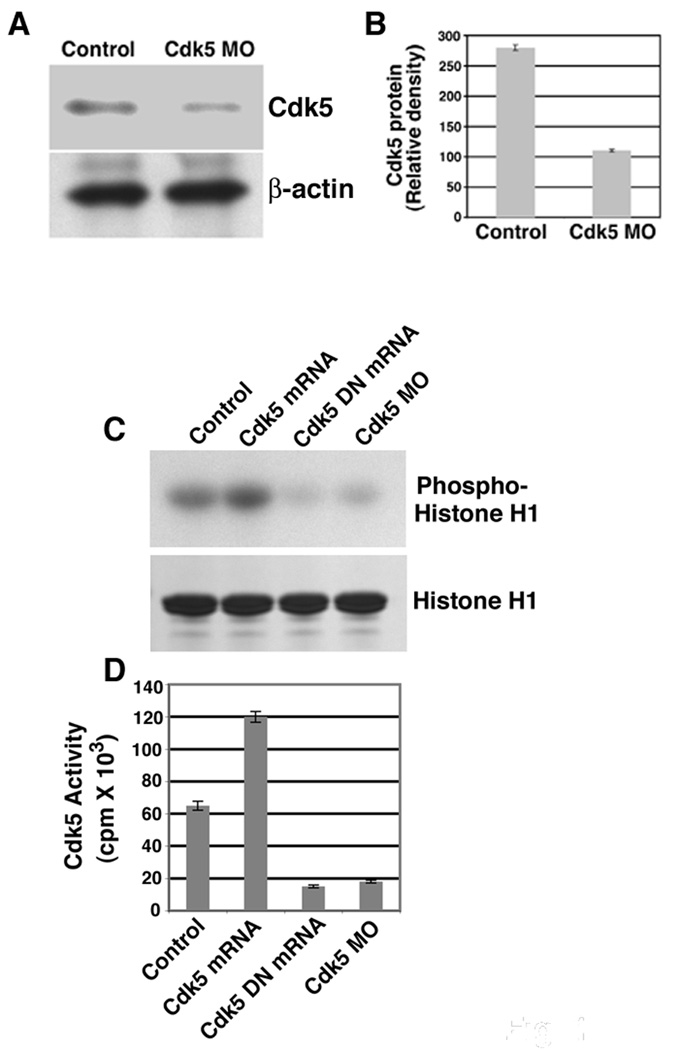
(A) Immunoblot of extracts prepared from 26 hpf embryos shows cdk5 protein levels in the control uninjected and cdk5 MO-injected embryos. Lower panel shows b-actin levels. Eighty micrograms of total protein was loaded on each lane. (B) Densitometric analyses of three different immunoblots as described in (A) shows significant loss of cdk5 protein in the cdk5 MO-injected embryo extracts. (C) Cdk5 activity in 26 hpf embryo extracts prepared from uninjected (control), cdk5 mRNA-injected (50 pg/embryo), kinase-dead human cdk5 (cdk5 DN) mRNA (50 pg/embryo), and cdk5 antisense morpholino oligonucleotide (cdk5 MO)-injected embryos. A representative autoradiograph of four separate experiments shows the phosphorylated histone H1 (upper panel) and Coomassie blue-stained histone H1 (bottom panel). (D) Relative levels of phosphorylated histone H1 (cpm) among the experimental groups derived from four separate experiments.
Cdk5 knockdown promotes motor neuron development
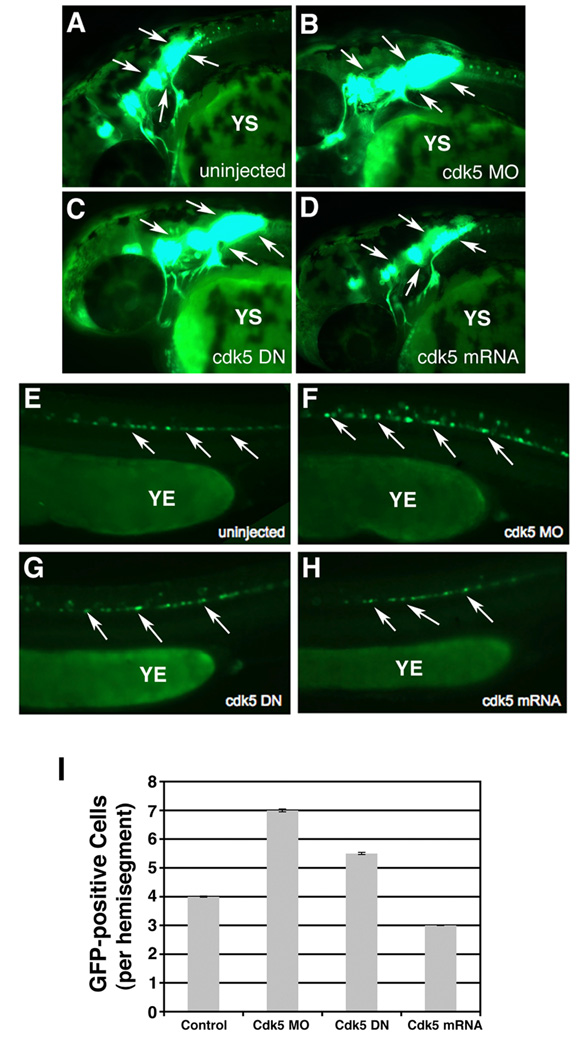
In our effort to determine whether cdk5 regulates motor neuron development, first we analyzed cdk5 MO-injected embryos at 26 hpf microscopically and morphologically. There was no morphological difference between the uninjected control and cdk5 MO-injected embryos (data not shown). However, compared to the control, uninjected embryos (n = 80, Fig. 2 A), all of the cdk5 MO-injected embryos (n = 80) displayed a significantly higher population of cranial as well as spinal motor neurons (Fig. 2B) as determined by the presence of GFP-positive neurons.
Figure 2. Cdk5 knockdown promotes cranial and spinal motor neuron development in 26 hpf embryos.
Images of live 26 hpf embryos that express islet-1 promoter-driven GFP in motor neurons. Lateral views of the anterior regions of the embryos show motor neurons in (A) uninjected control, (B) cdk5 morpholino (MO)-injected, (C) kinase-dead cdk5 (human) mRNA-injected and (D) zebrafish cdk5 mRNA-injected embryos. Arrows indicate the motor neuron populations in the brain. YS indicates yolk sac. Lateral views of the posterior region of the 26 hpf embryos show GFP-positive spinal motor neurons in, (E) uninjected control, (F) cdk5 morpholino (MO)-injected, (G) kinase-dead cdk5 (human) mRNA-injected (cdk5 DN), and (H) zebrafish cdk5 mRNA (50 pg injected/ embryo). YE indicates yolk extension. Arrows indicate some of the spinal motor neurons. The GFP-positive neurons or cells per each hemisegment are counted from 10 embryos in each group and the mean numbers are plotted with the standard error.
Next, we injected the mRNA transcribed from kinase-dead human cdk5 (cdk5 DN) and mRNA transcribed from zebrafish cdk5 (cdk5 mRNA) to 1- to 2-cell embryos. At 26 hpf, compared to the uninjected control (n = 80, Fig. 2 A), 100% of the cdk5 DN-expressing embryos (n = 80) showed significantly higher number of motor neurons, both cranial and spinal (Fig. 2 C). Conversely, as compared to the uninjected control embryos (n = 80, Fig. 2 A), over-expression of cdk5 (50 pg mRNA/embryo) suppressed the generation of motor neurons (80 of 80 embryos) (Fig. 2 D). Together, these results indicate that cdk5 negatively regulates motor neuron development in zebrafish.
Analysis of the spinal motor neurons revealed that compared to the uninjected embryos (Fig. 2 E), there was an excess of these GFP-positive neurons in the cdk5 MO-injected embryos (Fig. 2 F) and in cdk5 DN mRNA-injected embryos (Fig. 2 G). However, there were fewer motor neurons in cdk5-overexpressing embryos (Fig. 2 H) compared to the uninjected control. To quantify the difference in the number of the GFP-positive motor neurons, 10 embryos were taken from the control and cdk5 MO-injected groups and the GFP-positive neurons were counted from five hemisegments of each embryo, the first hemisegment being the one at the distal end of the yolk extension (YE). The mean values of the numbers of GFP-positive neurons were derived from the five hemisegments to project the number of these neurons per hemisegment. The results showed that there was a significant increase in the number of spinal motor neurons in the MO-injected embryos (7 cells/hemisegment) compared to that in the control, uninjected embryos (3.5 cells/hemisegment) (Fig. 2 I) indicating that loss of cdk5 activity promotes the development of the spinal motor neurons, while over-expression of cdk5 suppresses their development.
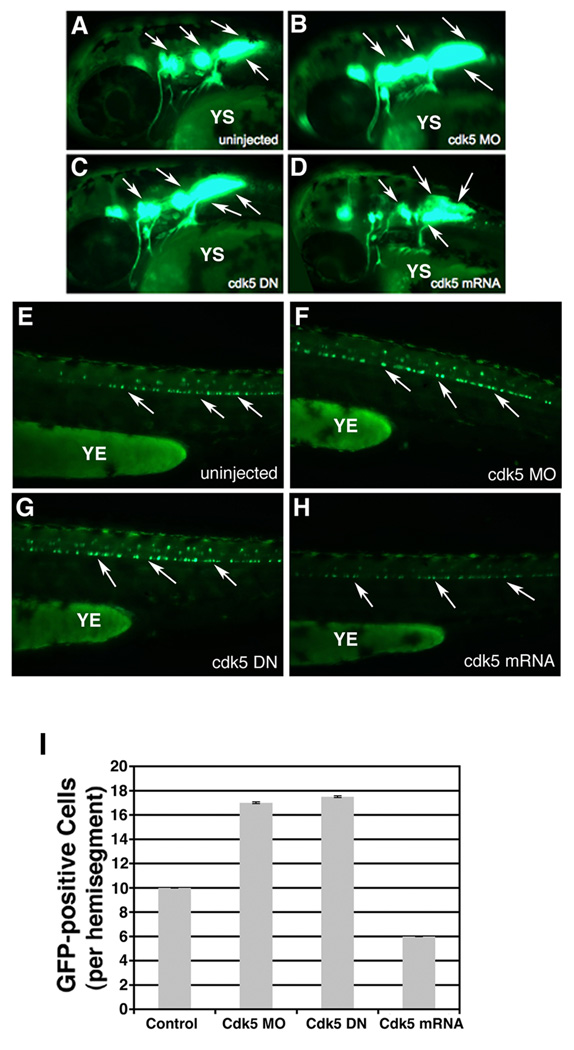
Through 48 h of development, compared to the control uninjected embryos (Fig. 3 A , E), an increase in both the cranial and spinal motor neuron population was observed in the cdk5 MO-injected embryos with no perturbation in the overall development (Fig. 3 B , F). In addition, compared to the uninjected embryos (Fig. 3 A , E), there was an increase in the cranial and spinal motor neurons in embryos injected with cdk5 DN (Fig. 3 C , G). The fewer motor neurons seen in cdk5-overexpressing embryos (Fig. 3 D , H) compared to the uninjected control were also observed at 48 hpf (Fig. 3 A , D). In the context of motor neuron development, while the uninjected control embryos had 10 cells/hemisegment, the kinase-dead cdk5-expressing embryos had 17 and the cdk5 mRNA-overexpressing embryos had only an average of 6 cells/hemisegment (Fig. 3 I).
Figure 3. Zebrafish embryos at 48 hpf continue to show over-production of motor neurons upon cdk5 knockdown.
Images of live 48 hpf islet 1:GFP transgenic fish. Lateral views of the anterior regions of the embryos show motor neurons in (A) uninjected control, (B) cdk5 morpholino (MO)-injected, (C) kinase-dead cdk5 (human) mRNA-injected and (D) zebrafish cdk5 mRNA (50)-injected embryos. Arrows indicate the motor neuron populations in the brain. YS indicates yolk sac. Lateral views of the posterior region of the embryos show GFP-positive spinal motor neurons in, (E) uninjected control, (F) cdk5 morpholino (MO)-injected, (G) kinase-dead cdk5 (human) mRNA-injected (cdk5 DN), and (H) zebrafish cdk5 mRNA (50 pg injected/ embryo). YE indicates yolk extension. Arrows indicate some of the spinal motor neurons. The GFP-positive neurons or cells per each hemisegment are counted from 10 embryos in each group and the mean numbers are plotted with the standard error (I).
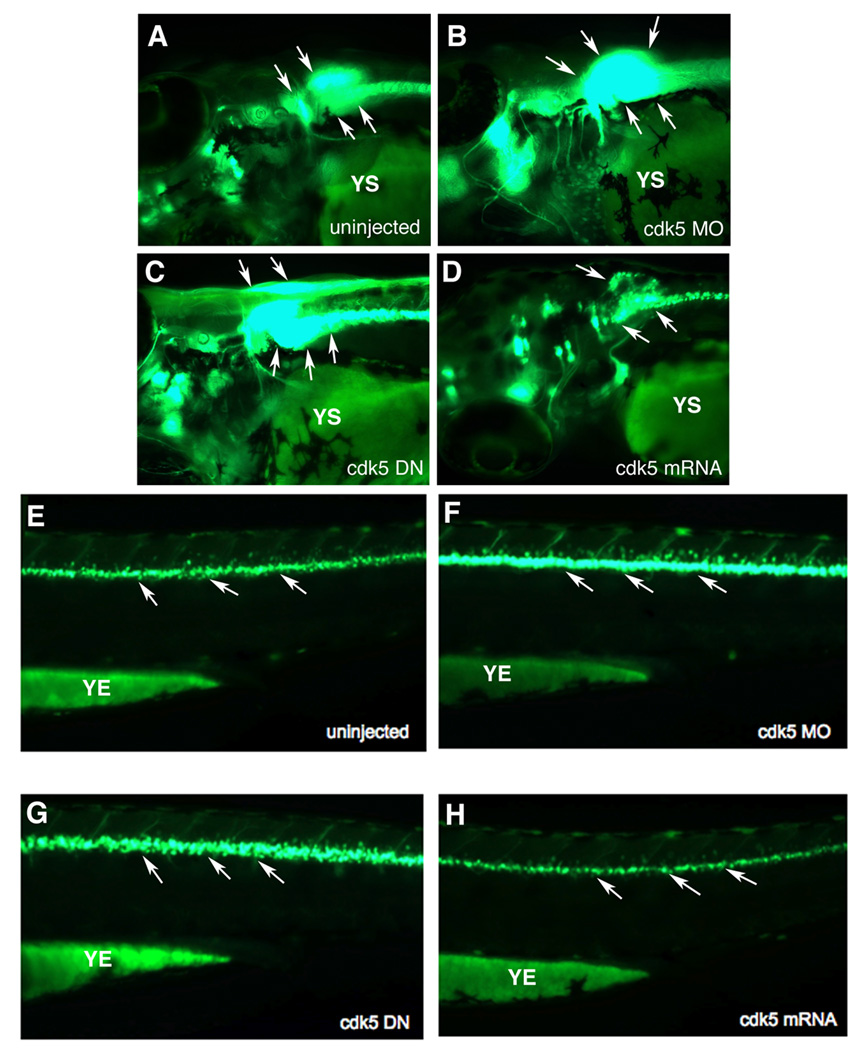
In 72 hpf embryos, the pattern of motor neuron development in response to cdk5 knockdown or over-expression remained unchanged as in the 26 and 48 hpf embryos. At 72 h an increase in the cranial motor neurons was observed in cdk5 MO-injected embryos compared to their uninjected control counterparts (Fig. 4 A , E). Over-expression of cdk5 DN resulted in an increase in the cranial motor neurons (Fig. 4 C) as compared to those in the uninjected control (Fig. 4 A), while embryos overexpressing cdk5 had less cranial motor neurons (Fig. 4 D). Cdk5 MO-injected (Fig. 4 F) as well as cdk5 DN-expressing embryos (Fig. 4 G) had an excess of spinal motor neurons compared to their uninjected counterparts (Fig. 4 E). However, in continuation of an earlier trend seen in 26 hpf embryos, there were fewer spinal motor neurons in cdk5 over-expressing embryos (Fig. 4 H) than in the uninjected control (Fig. 4 E). In these 3-day old embryos, spinal motor neurons were difficult to be counted as there were overlapping GFP signals due to an increased number of motor neurons. These results, consistent with the observations in 26 and 72 hpf embryos, demonstrate that loss of cdk5 activity is conducive to motor neuron development.
Figure 4. Effect of cdk5 knockdown on enhanced motor neuron generation in zebrafish embryos is retained at 72 hpf.
Images of live 72 hpf islet 1-GFP transgenic fish embryos. Lateral views of the anterior regions of the embryos show motor neurons in (A) uninjected control, (B) cdk5 morpholino (MO)-injected, (C) kinase-dead cdk5 (human) mRNA-injected, and (D) zebrafish cdk5 mRNA (50)-injected embryos. Arrowheads indicate the motor neuron populations in the brain. YS indicates the yolk sac. Lateral views of the posterior region of the embryos show GFP-positive spinal motor neurons in, (E) uninjected control, (F) cdk5 morpholino (MO)-injected, (G) kinase-dead cdk5 (human) mRNA-injected (cdk5 DN), and (H) zebrafish cdk5 mRNA (50 pg injected/ embryo). YE indicates the yolk extension. Arrows indicate spinal motor neurons.
Discussion
Our earlier studies demonstrated that siRNA-mediated cdk5 knockdown results in significantly fewer numbers of RB and trigeminal ganglia sensory neurons [11, 12, 16]. However, it was not clear if motor neurons were affected. Therefore, in order to specifically determine the role of cdk5 in motor neuron development, we used the islet-1 promoter-driven GFP transgenic (islet-1: GFP) zebrafish. In these fish, motor neurons specifically express GFP. Our results show that cdk5 MO injection and overexpression of a kinase-dead cdk5 mutant (cdk5 DN) independently promote motor neuron development. Cdk5 has been associated with neuronal differentiation. Loss of cdk5 during development leads to an ability of neurons to exit the cell cycle, coupled with their incomplete differentiation [17] and negatively affects neurite outgrowth, axonogenesis and neuronal differentiation in mammals and Drosophila [8, 9, 17–20]. In this study, we provide evidence that loss of cdk5 promotes motor neuron development. More confirmatory evidence comes from the suppressive effects of cdk5 over-expression achieved through microinjection of cdk5 mRNA, on the motor neuron development.
In adult zebrafish brain, significant neurogenesis takes place in specific neurogenic zones [21–24]. Although not to such a high degree, mammalian brains do have some neurogenic zones [25]. However, adult zebrafish spinal cord has very little ability for neurogenesis [24]. Differentiation of motor neurons in zebrafish and mammals is highly conserved as the specific transcription factors involved in motor neuron development (HB9 and islet-1/2) are found in the developing motor neurons of mammals [26, 27] and zebrafish [28, 29]. However, while lost neurons of the injured adult mammalian spinal cord are not replaced [30, 31], motor neuron regeneration occurs in lesioned adult zebrafish spinal cord as the zebrafish olig2-positive ependymo-radial glial cells retain the progenitor cell-like plasticity to become motor neurons [32]. Thus, we believe that our findings may enable us to understand the basis of motor neuron generation in mammals. Further analyses will be required in order to elucidate the mechanism by which cdk5 loss promotes motor neuron development. In conclusion, this is the first in vivo study to elucidate that cdk5 controls both cranial and spinal motor neuron generation.
Acknowledgements
This work was supported by NIH (NINDS) intramural funds. RR is a recipient of the NCI Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hartenstein V. Early neurogenesis in Xenopus: the spatio-temporal pattern of proliferation and cell lineages in the embryonic spinal cord. Neuron. 1989;3:399–411. doi: 10.1016/0896-6273(89)90200-6. [DOI] [PubMed] [Google Scholar]
- 2.Sasai Y, De Robertis EM. Ectodermal patterning in vertebrate embryos. Dev Biol. 1997;182:5–20. doi: 10.1006/dbio.1996.8445. [DOI] [PubMed] [Google Scholar]
- 3.Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human cdc2-related protein kinases. Embo J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 5.Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 6.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 7.Zheng M, Leung CL, Liem RK. Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J Neurobiol. 1998;35:141–159. doi: 10.1002/(sici)1097-4695(199805)35:2<141::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna HC, Pant RO, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 10.Becker CG, Lieberoth BC, Morellini F, Feldner J, Becker T, Schachner M. L1.1 is involved in spinal cord regeneration in adult zebrafish. J Neurosci. 2004;24:7837–7842. doi: 10.1523/JNEUROSCI.2420-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanungo J, Li BS, Goswami M, Zheng YL, Ramchandran R, Pant HC. Cloning and characterization of zebrafish (Danio rerio) cyclin-dependent kinase 5. Neurosci Lett. 2007;412:233–238. doi: 10.1016/j.neulet.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanungo J, Li BS, Zheng Y, Pant HC. Cyclin-dependent kinase 5 influences Rohon-Beard neuron survival in zebrafish. J Neurochem. 2006;99:251–259. doi: 10.1111/j.1471-4159.2006.04114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000;20:206–218. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 15.Li BS, Zhang L, Gu J, Amin ND, Pant HC. Integrin alpha(1) beta(1)-mediated activation of cyclin-dependent kinase 5 activity is involved in neurite outgrowth and human neurofilament protein H Lys-Ser-Pro tail domain phosphorylation. J Neurosci. 2000;20:6055–6062. doi: 10.1523/JNEUROSCI.20-16-06055.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanungo J, Zheng YL, Mishra B, Pant HC. Zebrafish Rohon-Beard Neuron Development: Cdk5 in the Midst. Neurochem Res. 2008 doi: 10.1007/s11064-008-9885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connell-Crowley L, Gall MLe, Vo DJ, Giniger E. The cyclin-dependent kinase Cdk5 controls multiple aspects of axon patterning in vivo. Curr Biol. 2000;10:599–602. doi: 10.1016/s0960-9822(00)00487-5. [DOI] [PubMed] [Google Scholar]
- 19.Smith D. Cdk5 in neuroskeletal dynamics. Neurosignals. 2003;12:239–251. doi: 10.1159/000074626. [DOI] [PubMed] [Google Scholar]
- 20.Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- 21.Adolf B, Chapouton P, Lam CS, Topp S, Tannhauser B, Strahle U, Gotz M, Bally-Cuif L. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol. 2006;295:278–293. doi: 10.1016/j.ydbio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Chapouton P, Adolf B, Leucht C, Tannhauser B, Ryu S, Driever W, Bally-Cuif L. her5 expression reveals a pool of neural stem cells in the adult zebrafish midbrain. Development. 2006;133:4293–4303. doi: 10.1242/dev.02573. [DOI] [PubMed] [Google Scholar]
- 23.Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol. 2006;295:263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Zupanc GK, Hinsch K, Gage FH. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J Comp Neurol. 2005;488:290–319. doi: 10.1002/cne.20571. [DOI] [PubMed] [Google Scholar]
- 25.Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 27.William CM, Tanabe Y, Jessell TM. Regulation of motor neuron subtype identity by repressor activity of Mnx class homeodomain proteins. Development. 2003;130:1523–1536. doi: 10.1242/dev.00358. [DOI] [PubMed] [Google Scholar]
- 28.Cheesman SE, Layden MJ, Ohlen TVon, Doe CQ, Eisen JS. Zebrafish and fly Nkx6 proteins have similar CNS expression patterns and regulate motoneuron formation. Development. 2004;131:5221–5232. doi: 10.1242/dev.01397. [DOI] [PubMed] [Google Scholar]
- 29.Park HC, Shin J, Appel B. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development. 2004;131:5959–5969. doi: 10.1242/dev.01456. [DOI] [PubMed] [Google Scholar]
- 30.Bareyre FM. Neuronal repair and replacement in spinal cord injury. J Neurol Sci. 2008;265:63–72. doi: 10.1016/j.jns.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Pinto L, Gotz M. Radial glial cell heterogeneity--the source of diverse progeny in the CNS. Prog Neurobiol. 2007;83:2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Reimer MM, Sorensen I, Kuscha V, Frank RE, Liu C, Becker CG, Becker T. Motor neuron regeneration in adult zebrafish. J Neurosci. 2008;28:8510–8516. doi: 10.1523/JNEUROSCI.1189-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]