Abstract
We have recently shown that the laminin-binding integrin receptor, α6β1, is prominently expressed in the developing chick retina, and its expression and activity are regulated during development on both retinal ganglion cells and other neural retinal cells. In the present study, we show that antibodies specific for the extracellular portion of the chick α6 subunit dramatically inhibit interactions in vitro between embryonic day 6 neural retinal cells and laminin, showing that α6β1 functions as an important laminin receptor on developing retinal neurons. In previous work, we showed that α6 mRNA levels on retinal ganglion cells decrease dramatically after E6 during the period that RGC axons innervate the optic tectum. In the present study, we show decreases in α6 mRNA are not prevented by ablation of the optic tectum, indicating that tectal contact is not the major cause of this decrease. Within the embryonic retina, the α6 subunit is codistributed, in part, with laminin, suggesting that it functions as a laminin receptor during retina development in vivo. Furthermore, two isoforms of the α6 protein with distinct cytoplasmic domains generated by differential splicing have quite different distribution patterns in the retina, suggesting that these two isoforms may have different functions during retinal development.
Keywords: laminin, integrin, retina, chick retina
INTRODUCTION
During the development of the vertebrate nervous system, each neuron has to find its way specifically to a final target where synapse formation occurs. In this process, the growth cones of developing neurons must recognize correct pathways reliably. The complex structure of the embryo makes it necessary for growth cones to respond to a diverse variety of cellular and extracellular substrata which contain the information needed to orient axonal outgrowth. Several classes of molecules have now been identified in the extracellular environment in vivo, which seem to be involved in promoting and guiding axons (reviewed by Dodd and Jessell, 1988; Jessell, 1988). Among these, several components of the extracellular matrix (ECM) seem likely to play important roles (reviewed by Sanes, 1989; Reichardt and Tomaselli, 1991). The best characterized receptors for ECM constituents are integrins, and several members of this family have been shown to be expressed on neurons and to promote neurite outgrowth on ECM-coated substrata in vitro (reviewed by de Curtis, 1991). Laminin (LN) is considered one of the most potent neurite outgrowth-promoting ECM molecules for several neuronal types in culture. Perhaps as many as 27 distinct isoforms of laminin exist as trimeric complexes of three subunits: an A homologue plus a B1 homologue plus a B2 homologue. The rapidly expanding family of identified laminin subunits consists at present of 8 polypeptides, three of which are homologues of the A subunit; two or three of which are B1 subunit homologues; and two of which are B2 chain homologues (cf. Sanes et al., 1990; Kallunki et al., 1992; O’Rear, 1992). While a few preliminary studies have been done characterizing neuronal interactions with partially purified preparations of other laminin isoforms, the most definitive studies to date have been done only with the first identified laminin isoform, which contains the A, B1 and B2 subunits (reviewed in de Curtis, 1991). In particular, studies in vitro have shown that embryonic day 6 (E6) neural retinal cells attach and spread on laminin, extending long neurites within 24 hours (Cohen et al., 1986; Hall et al., 1987). The effect of laminin on neurite outgrowth from E6 neural retinal cells and retinal ganglion cells can be completely abolished by the presence of monoclonal antibodies to the integrin β1 subunit (Cohen et al., 1986; Hall et al., 1987), implicating one or more β1-class integrin receptors in interactions of these cells with laminin. The presence of laminin along the vitreal surface of the embryonic retina and in the developing optic pathway suggests possible roles for this extracellular matrix protein during the development of the retina and primary visual projection (McLoon, 1984; Adler et al., 1985; Cohen et al., 1987; Halfter and Fua, 1987; McLoon et al., 1988).
Recently, we have shown that at least two potential LN-binding integrins, α3β1 and α6β1, are present in embryonic retina. The α6β1 heterodimer is expressed in E6 neural retinal cells and in a highly enriched preparation of retinal ganglion cells (de Curtis et al., 1991). By using antibodies and cDNA clones specific for the chick α6 subunit, we showed that the expression of this protein is down regulated between embryonic days 6 and 12 during the development of the chick neural retina, correlating with loss of ability of these neurons to interact with laminin. In particular, dramatic decreases in the levels of mRNA and protein were observed in retinal ganglion cells over this time span. The present study presents direct evidence, using inhibitory antibodies, that the α6β1 integrin functions as an important laminin receptor for retinal neurons. Colocalization of α6 with laminin in vivo suggests that the receptor is functional in vivo.
MATERIALS AND METHODS
Reagents and solutions
White Leghorn chicken eggs were purchased from Feather Hill Farm (Petaluma, CA). Restriction enzymes and Klenow fragment of DNA polymerase were from New England Biolabs (Beverly, MA) and Boehringer Mannheim Diagnostics, Inc. (Houston, TX). Sequence enzyme, reagents for sequencing and random hexanucleotide primers were from kits supplied by U. S. Biochemical Corp. (Cleveland, OH). [α-35S]dATP was from Amersham Corp. (Arlington Heights, IL). D-[6-3H(N)]-glucosamine hydrochloride and EN3HANCE were from NEN, DuPont Co. (Wilmington, DE). Protein A-sepharose CL-4B, CNBr-Sepharose CL-4B, and thiopropyl-Sepharose CL-4B were from Pharmacia LKB Biotechnology Inc. (Piscataway, NJ). Other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). Oligonucleotide primers and synthetic peptides were provided by facilities in the Howard Hughes Medical Institute at University of California at San Francisco. Laminin (LN) was purified from Engelbreth-Holm Swarm sarcoma as published (Timpl et al., 1979). Collagen IV was from Collaborative Research Inc. (Bedford, MA). Vectastain ABC kits for immunohistochemical staining were from Vector Laboratories (Burlingame, CA).
Antibodies
The polyclonal antibody against the chicken integrin β1 subunit was raised against the last 23 carboxy-terminal residues of this protein by Dr Kevin Tomaselli in this laboratory and is described by Tomaselli et al. (1988). Two peptides corresponding to carboxyl terminal portions of the two alternative cytoplasmic portions of the human α6 protein (Tamura et al., 1991) were synthesized at the Howard Hughes Medical Institute at University of California, San Francisco. The 35 amino acid-long cytoplasmic A peptide (CGFFKRNKKDHYDATYHKAEIHAQPSDKERLTSDA) and the 35 amino acid-long cytoplasmic B peptide, which contained an added amino-terminal cysteine not present in the α6-B sequence, (CRIRKEEREIKDEKYIDNLEKKQWITKWNRNESYS) were coupled to KLH through N-terminal cysteines (Calbiochem-Behring Corp., San Diego, CA). The conjugates were used to immunize rabbits, as described previously (de Curtis et al., 1991). In this paper, the antibodies raised against these two peptides were referred to as α6-cytoA and α6-cyto B antibodies, respectively. The purification and use of the α6- cytoA antibody has been previously described (de Curtis et al., 1991). The polyclonal antibody α6-EX was raised against a fusion protein containing the amino-terminal portion of the extracellular domain of the chicken α6 integrin subunit. A portion of the α6 protein corresponding to amino acids −16 to +514 (de Curtis et al., 1991) was cloned into the pAR3040 plasmid vector, at the BamHI site (Studier and Moffatt, 1986; Hoey and Levine, 1988). The final fusion protein expressed in BL21 (DE3)pLysS cells, contained an extra 14 amino acids at the amino-terminus and 16 amino acids at the carboxy-terminus derived from the plasmid. The fusion protein was used for immunization of rabbits after purification by SDS-PAGE and electroelution. IgG fractions from the serum were used for affinity purification of the antibody on a fusion protein-CNBr-Sepharose CL-4B column. A rabbit polyclonal antibody to mouse LN, named JW2, was prepared in this laboratory by Dr Janet Winter and was purified by affinity chromatography on LN as described (Lander et al., 1985). The monoclonal antibody CSAT was purified from ascites made by using hybridoma cells generously provided by Dr A. F. Horwitz, University of Pennsylvania, Philadelphia, PA. The monoclonal antibody against chick G4 was a kind gift of Dr F. Rathjen, Zentrum fur Molekulare Neurobiologie, Hamburg, Federal Republic of Germany.
Cell attachment assay and neurite outgrowth assay
E6 chick retinae were dissected and incubated for 6 minutes at 37°C in 0.1% trypsin (Worthington Biochemical Corp., Freehold, NJ) in Ca2+- and Mg2+-free PBS (CMF-PBS). Digestion was stopped by adding 0.2×volume of heat-inactivated fetal calf serum. Pellets were washed once in F12 nutrient mixture, and triturated in F12 containing 0.002% DNase I. For cell attachment, Linbro/Titer plates (Flow laboratories, Inc., Mc Lean, VA) were coated overnight with 20 µg/ml of LN or collagen IV in CMF-PBS. Coated and uncoated wells were incubated for 2 hours at room temperature with 1% BSA in CMF-PBS. Wells were washed twice with CMF-PBS and about 100,000 retinal cells were added to each well, after preincubation in a sterile tube for 20 minutes at room temperature in F12 medium with additives (5 µg/ml insulin, 30 nM selenium, 25 µg/ml human transferrin, 100 U/ml penicillin and streptomycin, according to Bottenstein et al., 1980). When indicated, preincubation occurred in the presence of α6-EX or NR IgG. Cells were sedimented at the bottom of the wells by centrifugation, and incubated for 1 hour at 37°C in 5% CO2 atmosphere in the same medium used during the preincubation. Unattached cells were removed by the brisk addition of warm medium followed by gentle vacuum suction. The cells were fixed with 3% paraformaldehyde, stained with Crystal Violet (0.5% in 20% methanol) and washed with water (Bodary et al., 1989). After solubilization with 1% SDS, A540 was measured in each well. The percentage of inhibition of cell attachment on LN or collagen IV was calculated as follows:
In each experiment every sample was in triplicate.
For neurite outgrowth assays, trypsinized retinal cells were prepared and preincubated with IgG as described above. About 30,000 cells were added to each well. Cells were cultured overnight at 37°C in a 5% CO2 atmosphere, and cultures were examined in the microscope for neurite outgrowth.
Metabolic labeling and immunoprecipitation
Aliquots of cell lysate prepared from dissociated E6 retinal cells metabolically labeled with [3H]glucosamine were used for immunoprecipitation with different anti-integrin antibodies. Preparation of cells, metabolic labeling, lysate preparation, and immunoprecipitation procedures were the same as described by de Curtis et al. (1991).
Immunohistochemical staining
Paraformaldehyde-fixed E6 and E12 chick retinae were embedded in O.C.T. (Miles Inc., Elkhart, IN) and frozen for sectioning. 16 µm cryosections were blocked for 1 hour at room temperature with 10% goat serum, 0.2% Triton X-100 in PBS. The sections were incubated for 1 hour at room temperature with 2.5 µg/ml preimmune or affinity purified immune IgG (α6-cytoA and α6-EX antibodies), preimmune or immune serum diluted 1:500 (α6-cytoB antibody), affinity-purified anti-LN IgG (JW2 polyclonal antibody, Lander et al., 1985), anti-chick LN IgG (a gift of Dr Charles Little) or 5 µg/ml IgG (monoclonal antibody G4). The sections were successively incubated with Vectastain ABC reagents (Vector Laboratories, Inc., Burlingame, CA) and developed to visualize HRP-catalyzed reaction product according to the supplier.
Tectal ablation and purification of retinal ganglion cells
Eggs were windowed at embryonic day 3 (E3) and allowed to continue to develop until E6. The optic tecta of E6 embryos were removed completely and embryos were allowed to develop until day E9–E11. In each experiment, a number of unoperated embryos were allowed to develop until E9–E11 in windowed eggs as controls. Retinal cells from operated and unoperated embryos between E9 and E11 were dissociated by incubation in hyaluronidase and trypsin and fractionated in Percoll gradients. 2–5 embryos were used for each sample. This procedure and characterization of retinal ganglion cell-enriched and depleted fractions are described in de Curtis et al. (1991). These two fractions, referred to as Fractions I and II, respectively in that paper, were used for RNA analysis.
RNA analysis
Total RNA was prepared as described in Chomczynski and Sacchi (1987). RNA electrophoresis, transfer to nitrocellulose, and detection with 32P-labeled α6 cDNA and actin cDNA probes was done as described in de Curtis et al. (1991). For photography, blots were exposed to Kodak XAR-5 film. Bands were quantitated on a Molecular Dynamics Computing Densitometer. Each value for α6 transcript was normalized to the corresponding value for β-actin transcript.
RESULTS
Characterization of three polyclonal antibodies raised against different portions of the α6 polypeptide
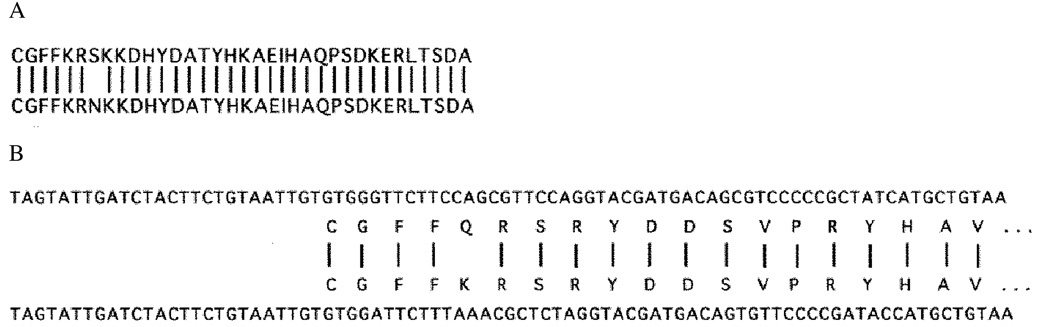
To study the possible functions of the chick α6β1 integrin in mediating interactions of retinal neurons with laminin in vitro and to characterize its distribution during retinal development in vivo, three polyclonal antibodies have been prepared to different portions of the α6 polypeptide. The α6-EX antibody was prepared to the external domain of α6, using a fusion protein. The α6-cytoA and α6-cytoB antibodies were prepared using as antigens the carboxy-terminal peptide sequences present in the cytoplasmic domains of two distinct variants of the human α6 subunit, which are generated by alternative RNA processing (Tamura et al., 1991). Fig. 1 shows the high degree of homology between the chick and human α6A cytoplasmic domains, where 34 of the 35 amino acid residues are conserved. Furthermore, analysis of the most 3′ portions of chick α6 cDNAs, sequenced by us previously (de Curtis et al., 1991), reveals the presence of nucleic acid residues which share 88% identity with the corresponding nucleic acid human sequence (Fig. 1B). The 3′ portion of this sequence contains a portion of an open reading frame encoding 19 amino acid residues that appears to correspond to a chick equivalent of the first portion of the 53-residue long human α6B cytoplasmic domain. 18 of these 19 residues are identical in the human and chick amino acid sequences, including several residues that are highly conserved in all integrin α subunits. These data suggested to us that two isoforms of the integrin α6 subunit are also present in the chick.
Fig. 1.
Sequence comparisons between human and chick cytoplasmic domains of integrin α6 proteins. In A, the entire amino acid sequences of the chick (top) and human (bottom) A class cytoplasmic domains are aligned. Note that 34 of 35 amino acid residues are identical. In B, the most 3′ sequenced portion of the chick α6 mRNA is aligned with the same region in the human α6 mRNA. Note the strong homology at the nucleic acid level. Note also that an open reading frame in the chick sequence (top) is very similar to an open reading frame in the human sequence (bottom), which has been shown to constitute the cytoplasmic domain of the B-isoform of the human α6 subunit (Tamura et al., 1991). Only the amino terminal portion of this reading frame is present in sequenced chick cDNAs and is aligned in this figure. Vertical lines indicate that identical amino acids that are present in each sequence.
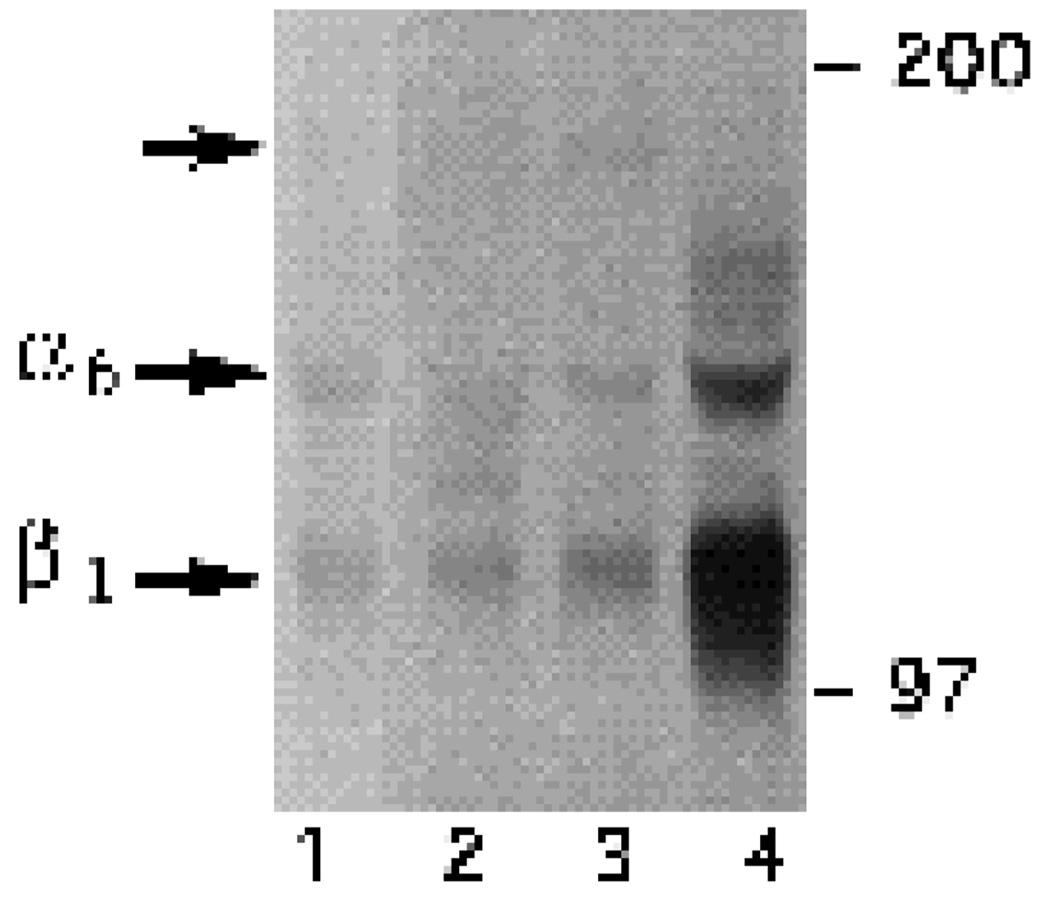
To obtain direct evidence for this possibility, the α6-EX, α6-cytoA and α6-cytoB antibodies were used to immuno-precipitate proteins from a lysate prepared from [3H]glucosamine-labeled E6 retinal neurons. SDS-PAGE analysis under non-reducing conditions of immunoprecipitates (Fig. 2) showed that all three antibodies recognize a band of 140×103 Mr, corresponding to the chick α6 integrin subunit (de Curtis et al., 1991). With all three antibodies the 140×103 Mr band coprecipitated with a polypeptide with a smaller apparent molecular mass, which comigrated with the integrin β1 subunit (Fig. 2, compare lanes 1–3 with lane 4). In addition, small amounts of a 180×103 Mr band were also coprecipitated by the α6-cytoB antibody (Fig. 2, lane 3). This band was not observed in immunoprecipitates using anti-β1 antibodies (Fig. 2, lane 4) and seems likely to be the integrin β4 subunit which has been shown to associate with α6 in other cells (e.g. Sonnenberg et al., 1990).
Fig. 2.
Immunoprecipitations from a cell lysate of metabolically labeled E6 retinal neurons. Cell lysates were prepared as described in the Experimental Procedures. For each lane, the same amount of TCA-precipitable radioactivity was used for immunoprecipitation with α6-EX (lane 1), α6-cytoA (lane 2), α6-cytoB (lane 3), or chick β1 (lane 4) polyclonal antibodies.
α6 is involved in the interactions of retinal cells with LN in vitro
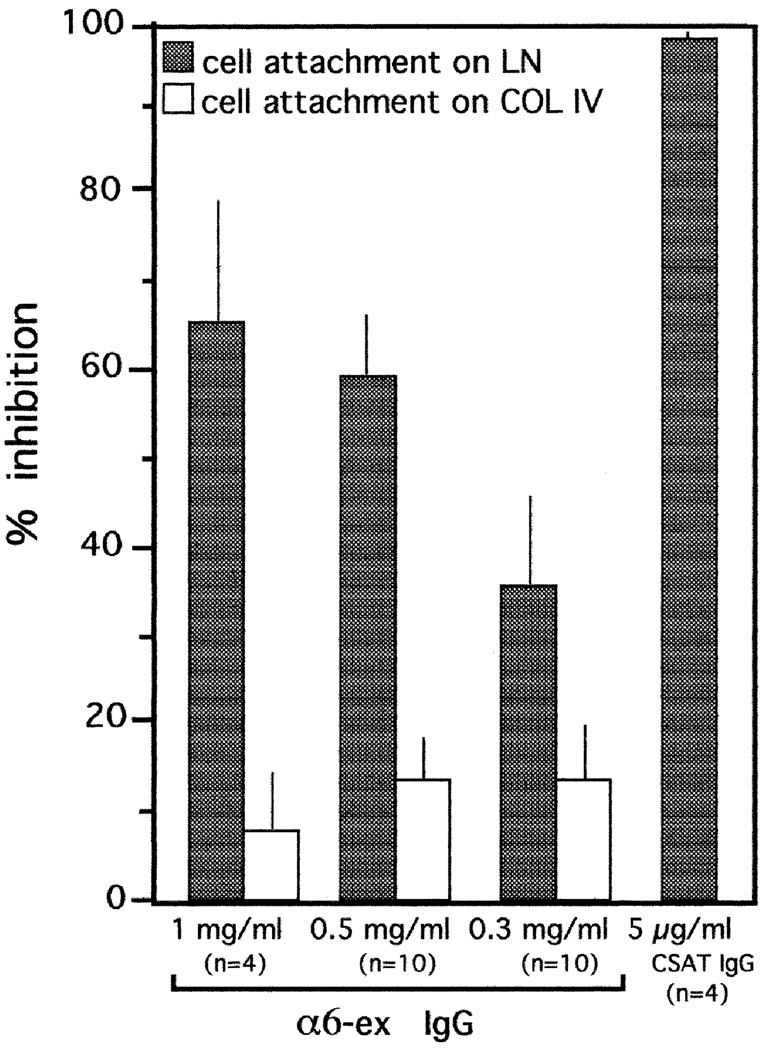
When E6 retinal neurons were plated onto LN-coated wells, cell attachment to the substratum was inhibited by the α6-EX antibody in a dose-dependent manner (Fig. 3). 66% of cell attachment was inhibited when 1 mg/ml of α6-EX IgG were present during a 1 hour incubation at 37°C. No significant inhibition was observed when the incubation was performed on collagen IV (70+/−6% inhibition) or when 1 mg/ml of normal rabbit (NR) IgG was present (not shown). An antibody to the integrin β1 subunit (CSAT) virtually eliminated attachment of E6 retinal neurons to LN during a 1 hour incubation, consistent with previous results (Hall et al., 1987; de Curtis et al., 1991).
Fig. 3.
Cell attachment assay of E6 retinal neurons on laminin (LN) or collagen IV (COL IV). Cells were prepared by trypsinization of E6 retinae and the cell attachment assay was performed for 1 hour at 37°C as described in the Experimental Procedures. Results of experiments with a function blocking β1 subunit-specific mAb (CSAT) and α6EX IgG are shown. In every experiment, each sample was tested in triplicate. Bars indicate the standard error; n represents the number of experiments.
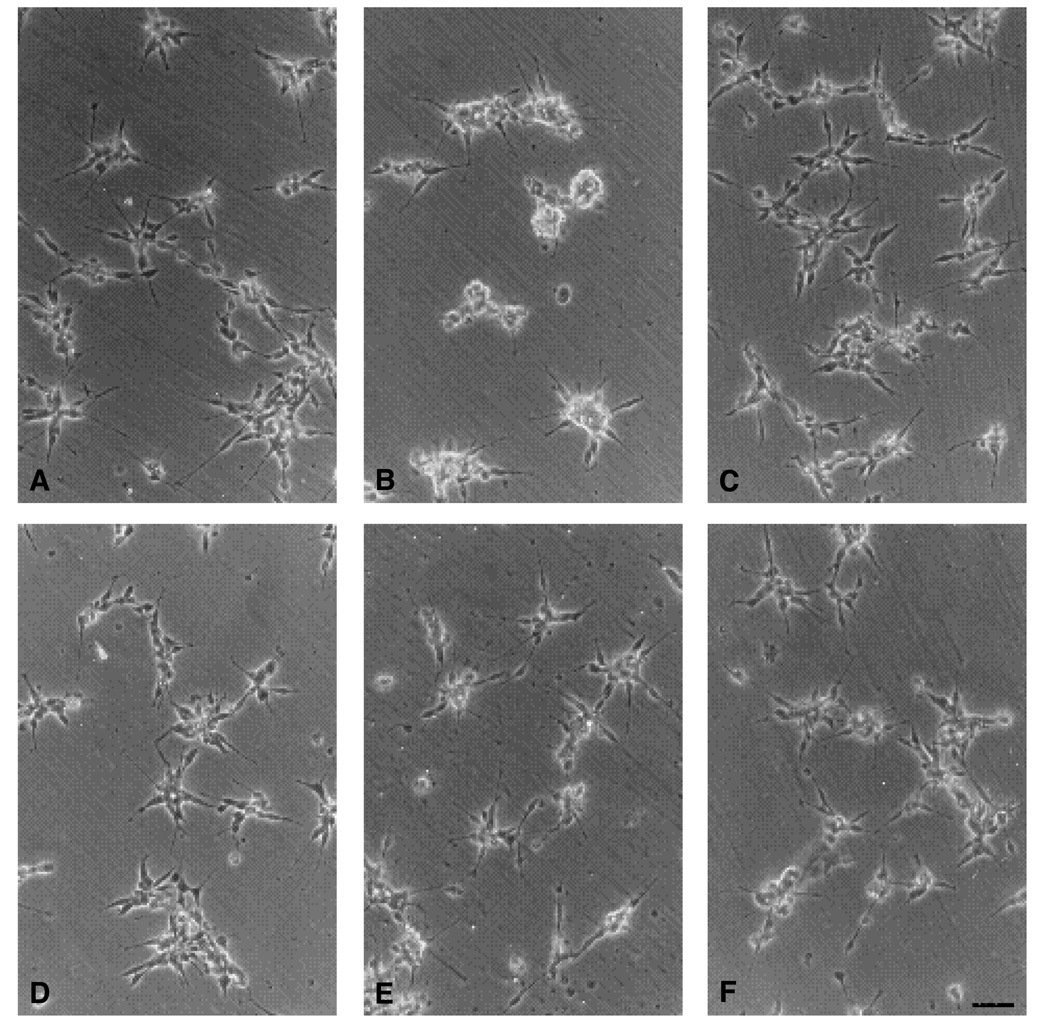
To test the effect of α6-EX antibody on neurite outgrowth, E6 retinal neurons were plated on LN or collagen IV in the presence of 0.5 mg/ml α6-EX IgG and cultured for 24 hours at 37°C. Results are quantitated in Table 1 and are illustrated in Fig. 4. Results in Table 1 show that fewer than half as many neurons developed neurites on laminin in the presence of α6EX IgG compared to control IgG or no IgG. As expected, α6EX IgG did not inhibit neurite formation on collagen IV. Previous results have shown that antibodies to the integrin β1 subunit almost completely eliminate retinal neurite formation on laminin and collagen IV (Hall et al., 1987). Although fewer cells extended neurites, data in Table 1 show that those neurons able to form neurites grew comparatively long neurites even in the presence of α6EX IgG. Taken together, the effects of α6EX IgG in cell adhesion and neurite outgrowth assays suggest that α6β1 is a prominent, but is not the only laminin receptor present on these neurons. The failure of α6EX IgG to reduce neurite length can be interpreted in many ways. For example, cell heterogeneity could explain these observations.
Table 1.
Effects of α6EX antibody on neurite formation and neurite outgrowth by retinal neurons
| Substrate | IgG | % cells with neurites |
Average neurite length |
|---|---|---|---|
| LN | − | 70 | 30.1±2.2 |
| LN | α6EX | 32 | 25.6±2.1 |
| LN | NR | 71 | 31.4±1.6 |
| col IV | − | 55 | 26.6±1.9 |
| col IV | α6EX | 55 | 26.8±1.9 |
| col IV | NR | 52 | 24.7±1.9 |
Dissociated E6 retinal neurons were plated on laminin (LN)- or collagen IV (col IV)-coated substrata and incubated for 24 hours at 37°C in culture medium plus no IgG (−), 0.5 mg/ml of α6EX IgG (α6EX) or 0.5 mg/ml of normal rabbit IgG (NR), as described in Materials and Methods. For quantitation of neurite formation by attached cells, 200 neurons in each culture condition were scored for the presence or absence of neurites. Results are presented as % of cells with neurons. For quantitation of neurite length, 50 neurons with neurites were examined in each culture condition. Neurite length is indicated as average neurite length ± standard error.
Fig. 4.
Neurite outgrowth of E6 retinal neurons on LN (A–C) or COL IV (D–F). Retinal neurons were prepared from E6 retinae and used in the neurite outgrowth assay as described in the Materials and methods. The cells were cultured overnight without IgG (A,D), with 0.5 mg/ml α6-EX IgG (B,E), or with 0.5 mg/ml normal rabbit IgG (C,F). Bar, 40 µm.
Differential localizations of two alternatively spliced forms of α6 protein in the developing chick retina
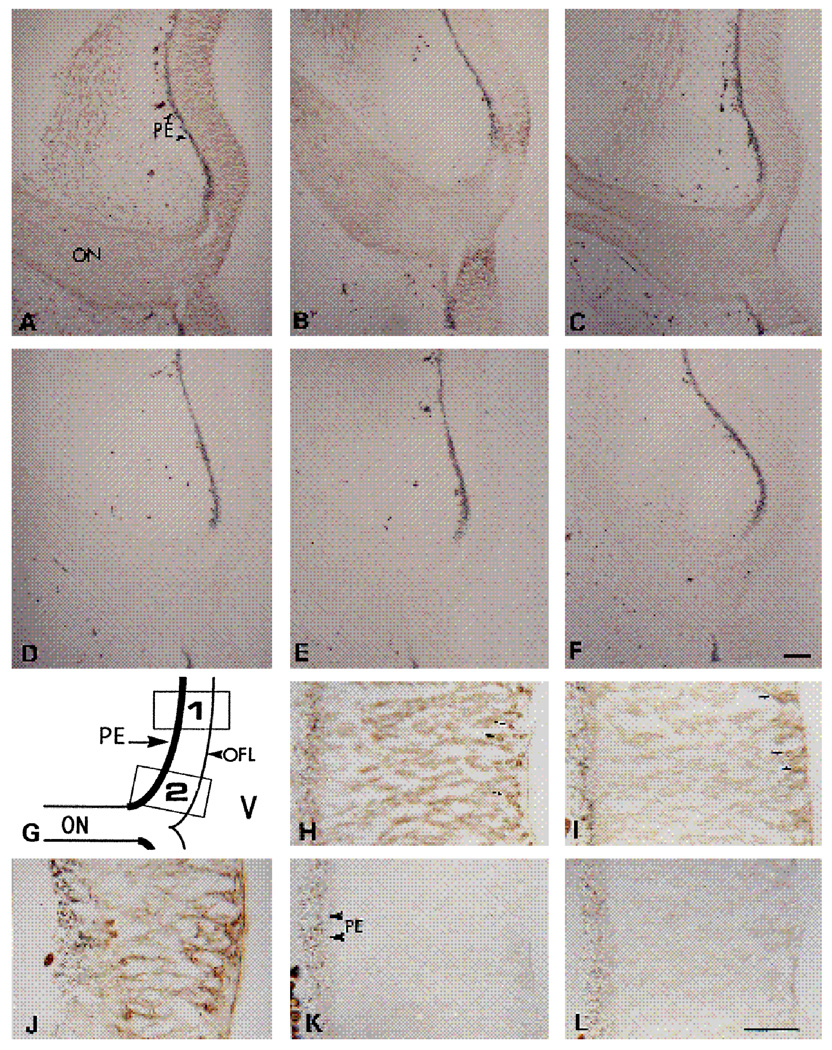
Cryosections of E6 retinae were stained with each of the three antibodies against the chick α6 subunit to study the localization of this LN receptor and its isoforms in the neural retina. At low magnification the α6-EX antibody showed a relatively uniform distribution of this integrin subunit through the width of the retina at E6 (Fig. 5A). Staining with the α6-cytoA and α6-cytoB antibodies revealed two dramatically different patterns of distribution of their respective antigens. The α6-cytoB antibody showed a reasonably uniform staining of cell surfaces throughout the E6 retina, which was weaker but similar to that observed when the α6-EX antibody was used (Fig. 5C). The α6-cytoA antibody stained an antigen restricted to a small region of the retina adjacent to the optic nerve and to the optic nerve itself (Fig. 5B). Sections incubated with each of the three preimmune sera showed very low backgrounds (Fig. 5D–F). At higher magnification the antigens recognized by the α6-EX and the α6-cytoB antibodies were detected throughout the retina (Fig. 5H,I). A discontinuous layer of cells characterized by spherical cell bodies located near the vitreal surface, developing retinal ganglion cells, showed more intense staining. At higher magnification, staining with the α6-cytoA antibody (Fig. 5K) was comparable to that obtained by using the corresponding preimmune serum (Fig. 5L), with the exception of the areas of the retina adjacent to the optic nerve (see diagram Fig. 5G, box 2), where an intense stain was detected throughout the thickness of the retina (Fig. 5J).
Fig. 5.
Micrographs of immunoperoxidase staining on E6 chick retina. 16 µm sections of E6 retina were incubated with α6-EX (A,H), α6-cytoA (B,J,K), or α6-cytoB (C,I) antibodies. D,E and Fare control stainings with preimmune IgG, for A,B and C, respectively. L is the control staining with preimmune IgG for J and K. G shows a diagram of the low power magnification of the retina as shown in panels A–F. Box 1 indicates the area of the retina shown in panels H,I,K and L. Box 2 indicates the area of the retina shown in J. ON, optic nerve; PE, pigmented epithelium; OFL, optic fiber layer; V, vitreous. Bars are 20 µm in A–F, 10 µm in H–L.
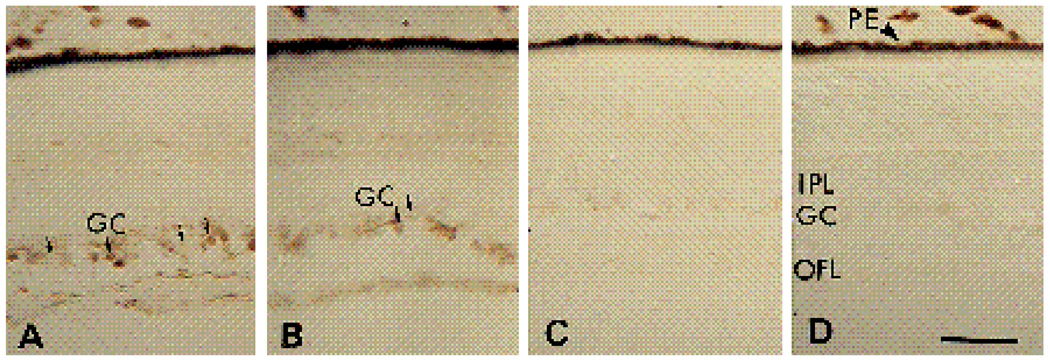
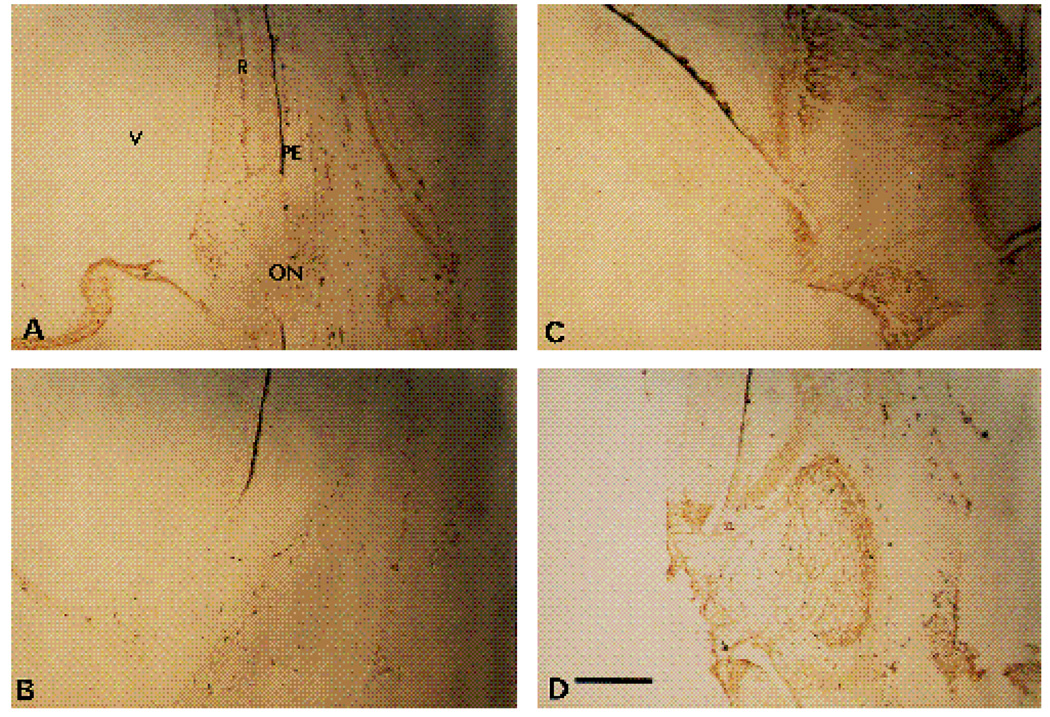
Similar patterns of staining were seen in sections of E12 retinae. In this case, both the α6-EX and the α6-cytoB antibodies strongly stained a small fraction of the cell bodies and axons present in the now recognizable ganglion cell layer (Fig. 6A,B). The majority of the spherical cell bodies in this layer showed no or weak staining. The intensities of the staining of most areas in the E12 chick retinae by the α6-cytoA antibody (Fig. 6C) were comparable to the staining obtained with the corresponding preimmune serum (Fig. 6D). In Fig. 7, the distribution of the α6 cyto A antigen is illustrated in low magnification photomicrographs of the retina and surrounding tissues adjacent to the optic nerve. Similar to the expression pattern in E6 retinae, specific staining with this antibody appeared to be restricted to areas of the neural retina around the optic nerve, and to the nearby vasculature protruding into the vitreous at this developmental stage.
Fig. 6.
Micrographs of immunoperoxidase staining on E12 chick retina. 16 µm sections were stained with α6-ex (A), α6-cytoB (B) and α6-cytoA (C) antibodies. D is a section stained with preimmune IgG for the α6-cytoA antibody. IPL, inner plexiform layer; OFL, optic fiber layer; PE, pigmented epithelium. Arrows point to examples of cells with strong or faint immunoperoxidase reaction product in the ganglion cell layer. Bar, 10 µm.
Fig. 7.
Low power micrographs of α6 and α6A expression patterns in E12 chick retina. 16 µm sections were stained with α6EX IgG (A), preimmune IgG for α6EX (B), or α6cytoA (C and D). The region surrounding the optic nerve is visible in each panel. ON, optic nerve; PE, pigment epithelium; V, vitreous. Reaction product is brown. Note localized distribution of α6A in retina adjacent to the optic nerve, within the optic nerve, and in tissues surrounding the retina. Bar, 10 µm.
Codistribution of the α6 polypeptide and LN in the developing chick retina
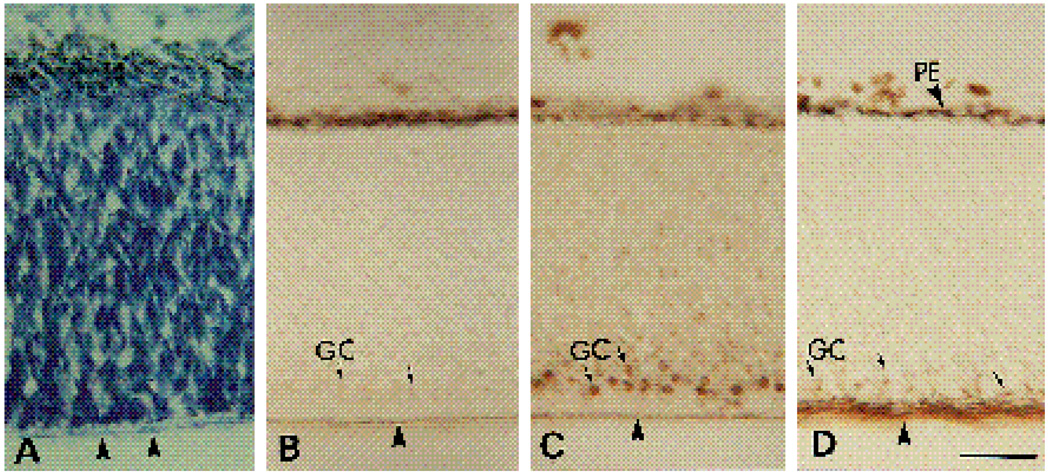
To determine whether the distribution of the α6 integrin subunit is consistent with this protein functioning as one subunit of a LN receptor (α6β1) in the developing chick retina, we compared the distribution of α6 with that of LN in E6 retinae. Parallel sections from E6 retinae were stained with the polyclonal anti-LN antibody JW2 (Fig. 8B), with α6-EX (Fig. 8C), or with the monoclonal antibody G4 which is specific for the cell adhesion molecule NgCAM (Fig. 8D). The G4 antibody has been shown to recognize specifically retinal ganglion cells in the developing chick retina where it is concentrated on their axons (Lemmon and Mc Loon, 1986). As expected, this antibody stained the layer of retinal ganglion cell axons running between the retina and the vitreous (Fig. 8D, arrowheads). When adjacent sections were incubated with the α6-EX antibody, a layer of round cell bodies colocalizing with Ng-CAM-expressing retinal ganglion cells was heavily stained in E6 retinae. Within the layer of axons projecting from retinal ganglion cells, α6 appeared concentrated on axons in close proximity to the basal lamina at the vitreal surface (Fig. 8C). A similar pattern of distribution was observed for LN, which was also concentrated in the basal lamina at the interface between the retina and the vitreous (Fig. 8B).
Fig. 8.
Micrographs of E6 (A–D) 16 µm thick retina sections stained with thionin (A) or with immunoperoxidase after incubation with anti-LN (B), α6-EX (C), or G4 (D) IgG. GC, ganglion cells (arrows); PE, pigmented epithelium. Arrowheads indicate the border between the optic fiber layer and the vitreous. Bar, 10 µm
Effects of target ablation on levels of α6 mRNA in retinal ganglion cells
Between E6 and E10, retinal ganglion cells lose responsiveness to laminin at a time correlating with innervation by their axons of the optic tectum (Cohen et al., 1989). These neurons also reduce by at least 4-fold α6 mRNA levels during this period of development (de Curtis et al., 1991). Tectal ablations appear to reverse, at least partially, the loss of laminin responsiveness (Cohen et al., 1989), making it seem possible that tectal innervation down regulates α6 mRNA levels.
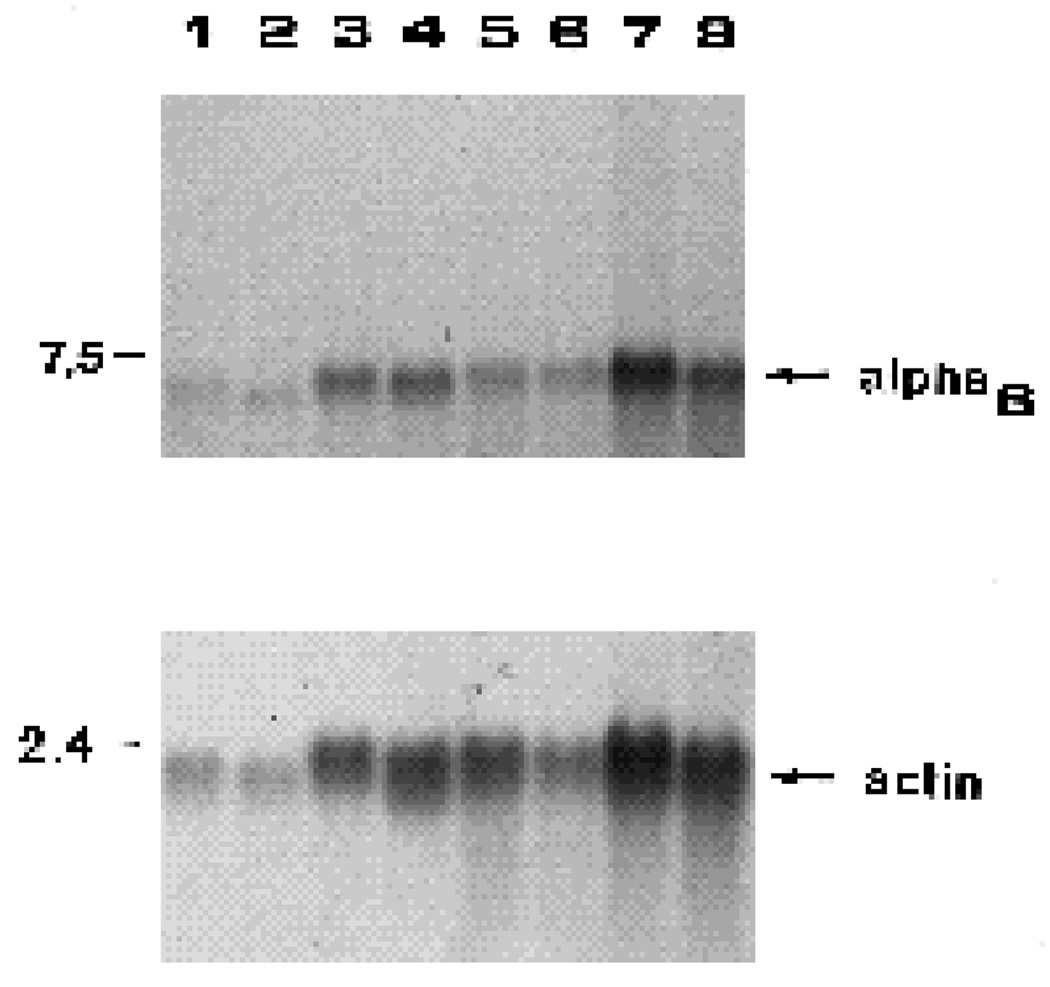
To determine whether α6 mRNA levels in retinal ganglion cells are regulated by innervation of the optic tectum, the optic tecta of E5-6 chicks were removed and mRNA levels examined in retinal neurons at E9–E11. RNA blots of total RNA from retinal ganglion cell-enriched and depleted fractions were assayed, using cells from unoperated chicks of the same ages as controls. Results in Fig. 9 show that a single α6 transcript of 5.3 kb was detected in control and tectal-ablated embryos, in agreement with previous observations (de Curtis et al., 1991). Ablation of the tectum at E5-6 did not appear to increase α6 mRNA levels dramatically in either retinal ganglion cells or other neuroretinal cells at E9-10. To quantitate these results more carefully, values for α6 mRNA levels were normalized to the corresponding values for β-actin mRNA, used as a standard in the RNA blots. The ratio of α6 mRNA levels in the experimental versus control animals is presented in Table 2. No significant change in α6 mRNA was seen in the fraction of retinal cells depleted of retinal ganglion cells. Individual experiments varied between a maximum 29% increase and minimum 16% decrease with an average of 0% change and a standard deviation of 20%. In contrast, a small average increase of approx. 20% was seen in retinal ganglion cells from operated animals. Individual experiments varied between a maximum 47% increase and minimum 24% decrease with a standard deviation of 33%. The levels in operated animals were not statistically significantly different from levels in controls. Since the level of α6 mRNA in E6 retinal ganglion cells is at least 300% higher than that in E12 retinal ganglion cells (de Curtis, 1991), one might have expected a similar magnitude increase in α6 mRNA in operated animals if the tectum provided the major signal down-regulating α6 mRNA in retinal ganglion cells. This thus seems unlikely.
Fig. 9.
Effects of tectal ablation on integrin α6 mRNA levels in retinal ganglion cells and other retinal cells. Retinal ganglion cells (RGC) and retinal ganglion cell depleted fractions (non-RGC) were used to prepare total RNA samples. Lanes 1–4 are RNA samples from one experiment; lanes 5–8 are RNA samples from a second experiment. RGC, controls (lanes 1,5); RGC, tectum-ablated (lanes 2,6); non-RGC, controls (lanes 3,7); non-RGC, tectum-ablated (lanes 4,8). RNA samples were fractionated by agarose gel electrophoresis, transferred to nitrocellulose and incubated with 32P-α6 probe. The upper section of this blot was incubated with the 32P-α6 probe, the lower section with the 32Pactin probe. The positions of DNA standards are indicated in kb on the left. When quantitated, tectal ablation resulted in 15% and 47% increases in α6 mRNA in the RGC fractions, and 11% and 16% decreases in the non-RGC fractions respectively in the two experiments depicted here.
Table 2.
Effect of tectal ablations on α6 mRNA levels in retinal ganglion cells (RGC) and cells depleted of retinal ganglion cells (non-RGC)
| α6 mRNA ablation | ||
|---|---|---|
| α6 mRNA control | ||
| Experiment | RGC | Non-RGC |
| 1 | 1.45 | 0.99 |
| 2 | 0.76 | 1.29 |
| 3 | 1.15 | 0.89 |
| 4 | 1.47 | 0.84 |
| average | 1.21±0.33 | 1.00±0.20 |
Retinal ganglion cells were separated from other cells on Percoll gradients as described in Materials and Methods. Each experiment used 3–5 embryos for each sample. The complete optic tecta were removed from embryos in windowed eggs at E5-6. Embryos were sacrificed between E9-11; total RNA was prepared and specific transcripts were quantitated by RNA blot as described in Materials and Methods. Values were normalized using β-actin mRNA as a control. Values in the table are ratios of normalized α6 mRNA levels in ablated versus control animals. Average values ± standard deviations are indicated.
DISCUSSION
Four major conclusions can be made from the results of this paper. First, the integrin α6β1 is a prominent, functional receptor for LN utilized by embryonic retinal neurons in vitro. Second, the presence of α6 antigen at contact sites between these neurons and LN in vivo suggests that this receptor is utilized in vivo. Third, striking differences in the spatial distributions of alternative variants of α6 suggest that there may be different functions for the two LN receptors, α6Aβ1 and α6Bβ1, in the developing retina. Finally, tectal ablation does not significantly affect α6 mRNA levels in retinal ganglion cells. Thus, previously observed effects of contact with optic tectum on laminin-responsiveness in retinal ganglion cells seem unlikely to reflect transcriptional regulation of α6 expression.
In previous work, survival and differentiation of neurons has been shown to be dramatically influenced by LN (reviewed in Sanes, 1989; Reichardt and Tomaselli, 1991). In particular, E6 retinal neurons have been shown to respond to LN-coated substrata by extending long neurites. In contrast, most E12 neural retinal cells in culture can neither adhere to nor extend neurites on LN (Cohen et al., 1986; Hall et al., 1987). The same authors have shown that β1 integrins are prominent receptors utilized by these neurons to interact with LN in vitro.
In a recent paper, we showed that the α6β1 integrin is one of the candidate LN receptors that is expressed in E6 retinal neurons, suggesting that it may be an important LN receptor for these cells (de Curtis et al., 1991). In the present study, we have provided direct evidence for this. An antibody, α6-EX, was prepared to a large fragment of the extra-cellular domain of the α6 subunit. This antibody was shown to recognize α6 specifically. In functional cell adhesion and neurite outgrowth assays, the α6-EX antibody strongly inhibits in a dose-dependent manner interactions of embryonic retinal neurons with LN, but not collagen IV. In agreement with previous work, antibodies to the β1 subunit were also shown to inhibit interactions of these neurons with LN. Since α6 and β1 coassociate in the embryonic neuroretina (de Curtis et al., 1991), the results imply that α6β1 is a functional receptor on these neurons for LN, but not collagen, consistent with its properties in other cells (Hall et al., 1990; Sonnenberg et al., 1990).
Since the inhibitory effects of α6-EX are strong, but incomplete, results in the present paper also suggest that an additional integrin functions as a LN receptor on retinal neurons. This receptor must contain the β1 subunit, since antibodies to β1 prevent virtually all interactions of these neurons with LN (this paper and Hall et al., 1987). Several other integrin α subunits - α1, α2, α3 and α7 - have now been shown to associate with β1 to form functional LN receptors. In previous work, we detected α3β1, but were not able to detect either α1β1 or α2β2 in the retina (de Curtis et al., 1991). By immunocytochemistry, though, α1 is clearly present in E7.5 retina and is reported to be expressed by retinal ganglion cells (Duband et al., 1992). Neither α1β1 nor α2β1 functions as laminin receptors in all cells in which they are expressed (cf. Chan and Hemler, 1993; Ignatius and Reichardt, unpublished). α1β1 also appears to recognize a site in laminin not recognized by retinal neurons (de Curtis et al., 1991). Thus, it may function as a retinal laminin receptor, but this is uncertain. In more recent work, we have not detected the α7 subunit in the embryonic neuroretina of the mouse, using immunocytochemistry (D. Sretavan and L. F. Reichardt, unpublished results), suggesting it is not likely to be present in the chick retina. Thus, the most likely candidate to be an additional LN receptor(s) in the embryonic chick retina is α3β1 (and possibly α1β1). If so, the activity of α3β1 must be regulated by post-translational mechanisms, since it is expressed by neurons at E12 that don’t interact strongly with LN (de Curtis et al., 1991).
To study the possible role of α6β1 as a LN receptor in vivo, we examined its expression during retinal development. As assessed using the α6-EX antibody, the α6 protein is broadly distributed in the retina at E6, with particularly high expression in cells close to the vitreal surface, where the retinal ganglion cells are developing at this stage (Thanos and Bonhoeffer, 1983; Rager, 1980). At this stage, retinal ganglion cell axons are growing along the vitreal surface, and many of them are entering the optic nerve, where they extend towards their target, the optic tectum (Rager, 1980). We found that the α6 polypeptide was most strongly localized to a subset of axons or perhaps domains of axons, those portions in contact with the vitreal surface, where it colocalized with the sites of highest levels of expression of LN. In contrast, NgCAM, a marker for the axons of mammalian and chick retinal ganglion cells (Lemmon and McLoon, 1986; Pigott and Davies, 1987), was expressed at high levels throughout the entire width of the retinal ganglion cell axon tract consistent with its previously demonstrated role in axon-axon interactions (cf. Chang et al., 1987).
In E12 retinas, the overall distribution of the α6-EX antigen was more restricted. Fewer cells still expressed high levels of this protein (Fig. 6). At this stage of development, virtually all retinal ganglion cells have reached the optic tectum. The layer of retinal ganglion cell axons between the retina and the laminin-rich vitreous is thicker at this stage, but only domains of axons in proximity to the laminin-rich vitreal surface showed a high level of expression of the α6 subunit (Fig. 6). The correlation between the patterns of distribution of α6 and LN in vivo, together with the demonstrated role of α6β1 as LN receptor in retinal cells in vitro strongly suggest that this integrin is an important LN receptor in the developing chick retina. Numerous previous studies on non-neuronal cells have documented many examples where integrins are colocalized on domains of plasmalemma in contact with their ligands (cf. Carter et al., 1990a,b).
Possible functions of laminin mediated through this receptor include regulation of migration or morphogenesis of neurons in early retinal development at times when both laminin and α6β1 are expressed at moderate levels throughout the width of the retina. Isoforms of laminin have also been detected along virtually the entire embryonic retinal-tectal pathway (McLoon, 1984; McLoon et al., 1988; Adler et al., 1985; Cohen et al., 1987; Halfter and Fua, 1987). Expression of laminin is transient in the optic stalk and correlates with the ability of retinal ganglion cells to utilize laminin as a substratum (cf. Cohen et al., 1987; 1989). These observations suggest that laminin and the α6β1 integrin will prove to be important in regulating development and axon outgrowth of retinal ganglion cells. It should now be possible to test these possible functions in vivo using α6-specific inhibitory antibodies and other appropriate reagents.
The present paper presents evidence that α6 antibodies fail to stain the majority of retinal ganglion cells in E12 neuroretina. Previously, analysis of the expression of the α6 integrin subunit at the mRNA and polypeptide levels has shown a dramatic decrease in the expression of this mRNA and protein between E6 and E12 in retinal ganglion cells (de Curtis et al., 1991). This suggests that transcriptional regulation of the expression of α6 accounts, at least in part, for the decreased responsiveness of older retinal ganglion cells to LN (de Curtis et al., 1991). Since significant α6 protein is seen on a proportion of these neurons, though, transcriptional regulation does not appear to be sufficient to account for the loss of responsiveness in neurite outgrowth assays of essentially all retinal ganglion cells neurons to laminin. As will be discussed below, the ligand-binding activity of integrins has been shown to be regulated on other neurons in the retina (Neugebauer and Reichardt, 1991) and may potentially be reduced also in older retinal ganglion cells. Alternatively, other developmental changes may reduce the signals transmitted by laminin binding to α6β1 which result in neurite outgrowth.
Tectal ablation has been reported to prevent partially the decrease in responsiveness of older retinal ganglion cells to laminin (Cohen et al., 1989), suggesting that target contact may regulate integrin expression in these cells. In the present paper, we have attempted to determine whether contact with the optic tectum regulates α6 expression at the mRNA level by examining effects of tectal ablation at E5-6 on α6 mRNA levels at E9-11. In normal animals during approximately this interval, α6 mRNA levels in retinal ganglion cells are reduced to less than one-fourth of the E6 levels (de Curtis et al., 1991). This is not prevented by ablation of the tectum (Fig. 9 and Table 2). Thus, tectal contact does not appear necessary for down-regulation of α6 mRNA. The report that tectal ablation partially prevents the decrease in laminin-responsiveness of retinal ganglion cells (Cohen et al., 1989) could be explained by effects on expression of other laminin receptors, such as α1β1 or α3β1. Alternatively, the ligand-binding activity of residual α6β1 receptors on retinal ganglion cells may be activated by tectal ablation.
Results in the present paper demonstrate changes in distribution and reductions in apparent levels of α6 subunit expression in most areas of the developing retina (see Fig 6, Fig 7, Fig 8). In previous work, using protein and RNA blots, dramatic decreases in expression in non-retinal ganglion cell populations were not seen (de Curtis et al., 1991), even though these cells at E12 have lost the abilities to adhere to or extend neurites on laminin (containing the A, B1 and B2 subunits). Our previous work also demonstrated that substantial levels of the α6β1 receptor were present on the surfaces of older retinal cells fractionated to remove retinal ganglion cells. Taken together, the results suggest that α6β1 is present in a comparatively inactive state on the surfaces of these cells. Consistent with this possibility, binding assays indicate that the affinity of laminin-binding sites for laminin is reduced approx. 100-fold on the neurites of E9 compared to E6 retinal cells (Cohen et al., 1989). In addition, a monoclonal antibody, TASC, that binds the β1 subunit and activates ligand-binding by β1-integrins restores the ability of E12 retinal neurons to bind to laminin (Neugebauer and Reichardt, 1991). Posttranslational activation of the surface α6β1 receptor has been shown previously in macrophages (Shaw et al., 1990). These results suggest that a large fraction of the α6β1 receptor present in E12 neuroretina is not active, but can be activated by appropriate physiological stimuli, which remain to be identified.
Alternative RNA processing, including in some cases alternative exon splicing, has been described for some integrin subunits, and more recently variants with alternative cytoplasmic domains generated by differential RNA processing have been described for the mammalian α3 and α6 subunits (Tamura et al., 1991). By using antibodies specific for the two alternative cytoplasmic domains of the α6 subunit, we found that both forms were present in the developing chick neural retina, and that the patterns of distribution of the two α6 isoforms were quite different. The α6-cytoA subunit was detected in a very restricted distribution, with high levels only near the optic nerve, while the distribution of the α6-cytoB isoform was virtually identical to that of the α6 subunit in toto, as detected using the α6-EX antibody. In previous work, the two different α6 isoforms have been shown to be expressed differentially in different tissues and during differentiation using PCR analysis (Tamura et al., 1991; Cooper et al., 1991). The present paper presents the first evidence for differential distribution of these isoforms within an organ or tissue. Since the sequence of each of the alternatively expressed cytoplasmic tails is conserved in mammalian and avian species, each seems likely to have a distinct function. As one possibility, different cytoplasmic domains on the α6 subunit might allow a single ECM protein, laminin, to transmit different signals to different cells.
In summary, the results presented in this paper show that the α6β1 receptor is a prominent LN receptor for retinal neurons, and suggest roles for the α6β1 integrin during retinal development and retinal ganglion cell axonogenesis. It will be important to analyze in the future the exact role of this and other integrins in mediating retinal development in vivo, and to see whether its different isoforms have more specific roles in regulating neuronal differentiation.
Acknowledgments
We are grateful to Kristine Venstrom for her help with the preparation and purification of the α6-cytoA and α6-cytoB antibodies, and to Dr Vito Quaranta (Department of Immunology, Research Institute of Scripps Clinic, La Jolla, California 92037) for providing us with the sequence of the human α6-cytoB peptide. We also would like to acknowledge the generous gift of the G4 monoclonal antibody (anti-NgCAM) from Dr F. Rathjen (Zentrum fur Molekulare Neurobiologie, Hamburg, Federal Republic of Germany), the CSAT monoclonal antibody (anti-α1) from Dr A. F. Horwitz (University of Pennsylvania, Philadelphia, PA)., anti-EHS-laminin antibodies (JW2) from Janet Winter, and anti-chick laminin antibodies from Dr Charles Little (University of Virginia, Charlottesville, VA). We thank Dr Frances Lefcort for helpful comments on the manuscript.
This work was supported by the National Institutes of Health grant 19090 to L. F. Reichardt. I. de Curtis was supported by a fellowship from the Muscular Dystrophy Association. L. F. Reichardt is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- CMF-PBS
Ca2+- and Mg2+-free PBS
- ECM
extracellular matrix
- E6
embryonic day 6
- E12
embryonic day 12
- LN
laminin
- NR
normal rabbit
References
- Adler R, Jerdan J, Hewitt AT. Responses of cultured neural retinal cells to substratum-bound laminin and other extracellular matrix molecules. Dev. Biol. 1985;112:100–114. doi: 10.1016/0012-1606(85)90124-1. [DOI] [PubMed] [Google Scholar]
- Bodary SC, Napier MA, McLean JW. Expression of recombinant platelet glycoprotein IIb/IIIa results in a functional fibrinogen-binding complex. J. Biol. Chem. 1989;264:18859–18862. [PubMed] [Google Scholar]
- Bottenstein JE, Skaper SD, Varon S, Sato J. Selective survival of neurons from chick embryo sensory ganglionic dissociates by use of a defined, serum-free medium. Exp. Cell Res. 1980;125:183–190. doi: 10.1016/0014-4827(80)90202-5. [DOI] [PubMed] [Google Scholar]
- Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins α3β1 in focal adhesions and α6β4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: Relation to hemidesmosomes. J. Cell Biol. 1990a;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter WG, Wayner EA, Bouchard TS, Kaur P. The role of integrins α2β1 and α3β1 in cell-cell and cell-substrate adhesion of human epidermal cells. J. Cell Biol. 1990b;110:1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BMC, Hemler ME. Multiple functional forms of the integrin VLA-2 can be derived from a single α2 cDNA clone: Interconversion of forms induced by an anti-β1 antibody. J. Cell Biol. 1993;120:537–543. doi: 10.1083/jcb.120.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Rathjen FG, Raper JA. Extension of neurites on axons is impaired by antibodies against specific neural cell surface glycoproteins. J. Cell Biol. 1987;104:355–362. doi: 10.1083/jcb.104.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen J, Burne JF, Winter J, Bartlett PF. Retinal ganglion cells lose response to laminin with maturation. Nature. 1986;322:465–467. doi: 10.1038/322465a0. [DOI] [PubMed] [Google Scholar]
- Cohen J, Burne JF, McKinlay C, Winter J. The role of laminin and the laminin/fibronectin receptor complex in the outgrowth of retinal ganglion cell axons. Dev. Biol. 1987;122:407–418. doi: 10.1016/0012-1606(87)90305-8. [DOI] [PubMed] [Google Scholar]
- Cohen J, Nurcombe V, Jeffrey P, Edgar D. Developmental loss of functional laminin receptors on retinal ganglion cells is regulated by their target tissue, the optic tectum. Development. 1989;107:381–387. doi: 10.1242/dev.107.2.381. [DOI] [PubMed] [Google Scholar]
- Cooper HM, Tamura RN, Quaranta V. The major laminin receptor of mouse embryonic stem cells is a novel isoform of the α6β1 integrin. J. Cell Biol. 1991;115:843–850. doi: 10.1083/jcb.115.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I. Neuronal interactions with extracellular matrix. Curr. Opin. Cell Biol. 1991;3:824–831. doi: 10.1016/0955-0674(91)90056-5. [DOI] [PubMed] [Google Scholar]
- de Curtis I, Quaranta V, Tamura RN, Reichardt LF. Laminin receptors in the retina: sequence analysis of the chick integrin α6 subunit. Evidence for transcriptional and posttranslational regulation. J. Cell Biol. 1991;113:405–416. doi: 10.1083/jcb.113.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Jessell TM. Axon guidance and the patterning of neuronal projections in vertebrates. Science. 1988;242:692–699. doi: 10.1126/science.3055291. [DOI] [PubMed] [Google Scholar]
- Duband J-L, Belkin AM, Syfrig J, Thiery JP, Koteliansky VE. Expression of α1 integrin, a laminin-collagen receptor, during myogenesis and neurogenesis in the avian embryo. Development. 1992;116:585–600. doi: 10.1242/dev.116.3.585. [DOI] [PubMed] [Google Scholar]
- Halfter W, Fua CS. Immunohistochemical localization of laminin, neural cell adhesion molecule, collagen type IV and T- 61 antigen in the embryonic retina of the japanese quail by in vivo injection of antibodies. Cell Tissue Res. 1987;249:487–496. doi: 10.1007/BF00217320. [DOI] [PubMed] [Google Scholar]
- Hall DE, Neugebauer KM, Reichardt LF. Embryonic neural retinal cell response to extracellular matrix proteins: developmental changes and effects on the cell substratum attachment antibody (CSAT) J. Cell Biol. 1987;104:623–634. doi: 10.1083/jcb.104.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, Sonnenberg A, Damsky CH. The α1β1 and α6β1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J. Cell Biol. 1990;110:2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey T, Levine M. Divergent homeo box proteins recognize similar DNA sequences in Drosophila. Nature. 1988;332:858–861. doi: 10.1038/332858a0. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Adhesion molecules and the hierarchy of neural development. Neuron. 1988;1:3–13. doi: 10.1016/0896-6273(88)90204-8. [DOI] [PubMed] [Google Scholar]
- Kallunki P, Sainio K, Eddy R, Byers M, Kallunki T, Saviola H, Beck K, Hirvonen H, Shows T, Tryggvason K. A truncated laminin chain homologous to the B2 chain: Structure, spatial expression and chromosomal assignment. J. Cell Biol. 1992;119:679–693. doi: 10.1083/jcb.119.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD, Fujii DK, Reichardt LF. Laminin is associated with the neurite outgrowth promoting factor found in conditioned media. Proc. Natl. Acad. Sci. U. S. A. 1985;82:2183–2187. doi: 10.1073/pnas.82.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon V, Mc Loon SC. The appearance of an L1-like molecule in the chick primary visual pathway. J. Neurosci. 1986;6:2987–2994. doi: 10.1523/JNEUROSCI.06-10-02987.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoon SC. Development of the retinotectal projection in chicks. In: Sharma SC, editor. Organizing Principles of Neural Development. New York: Plenum Press; 1984. pp. 325–342. [Google Scholar]
- McLoon SC, McLoon LK, Palm SL, Furcht LT. Transient expression of laminin in the optic nerve of the developing rat. J. Neurosci. 1988;8:1981–1990. doi: 10.1523/JNEUROSCI.08-06-01981.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer KM, Reichardt LF. Cell-surface regulation of β1-integrin activity on developing retinal neurons. Nature. 1991;350:68–71. doi: 10.1038/350068a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rear JJ. A novel laminin B1 chain variant in avian eye. J. Biol. Chem. 1992;267:20555–20557. [PubMed] [Google Scholar]
- Pigott R, Davies AM. The monoclonal antibody 69A1 recognizes an epitope found on neurones with axons that fasciculate but not on those with non-fasciculating processes. Development. 1987;100:489–500. doi: 10.1242/dev.100.3.489. [DOI] [PubMed] [Google Scholar]
- Rager GH. Development of the retinotectal projection in the chicken. Adv. Anat. Embryol. Cell Biol. 1980;63:1–92. [PubMed] [Google Scholar]
- Reichardt LF, Tomaselli KJ. Extracellular matrix molecules and their receptors: functions in neural development. Annu. Rev. Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR. Extracellular matrix molecules that influence neural development. Annu. Rev. Neurosci. 1989;12:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Engvall E, Butkowski R, Hunter DD. Molecular heterogeneity of basal laminae: Isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J. Cell Biol. 1990;111:1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Messie JM, Mercurio AM. The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the α6β1 integrin. J. Cell Biol. 1990;110:2167–2174. doi: 10.1083/jcb.110.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A, Linders CJT, Modderman PW, Damsky CH, Aumailley M, Timpl R. Integrin recognition of different cell-binding fragments of laminin (P1,E3, E8) and evidence that α6β1 but not α6β4 functions as a major receptor for fragment E8. J. Cell Biol. 1990;110:2145–2155. doi: 10.1083/jcb.110.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tamura RN, Cooper HM, Collo G, Quaranta V. Cell type-specific integrin variants with alternative α chain cytoplasmic domains. Proc. Natl. Acad. Sci. USA. 1991;88:10183–10187. doi: 10.1073/pnas.88.22.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos S, Bonhoeffer F. Investigations on the development and topographic order of retinotectal axons: anterograde and retrograde staining of axons and perikarya with rhodamine in vivo. J. Comp. Neurol. 1983;219:420–430. doi: 10.1002/cne.902190404. [DOI] [PubMed] [Google Scholar]
- Timpl R, Rhode H, Gehron-Robey P, Rennard S, Foidart J-M, Martin G. Laminin, a glycoprotein from basement membranes. J. Biol. Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- Tomaselli KJ, Damsky CH, Reichardt LF. Purification and characterization of mammalian integrins expressed by a rat neuronal cell line (PC 12): evidence that they may function as α/β heterodimeric receptors for collagen IV and laminin. J. Cell Biol. 1988;107:1241–1252. doi: 10.1083/jcb.107.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]