Abstract
Objective
Medial and lateral compartment bone marrow lesions (BMLs) have been tied to cartilage loss. We undertook this study to assess 2 types of BMLs in the central region of the knee (type 1 BMLs, which are related anatomically to anterior cruciate ligament [ACL]/posterior cruciate ligament [PCL] insertions, and type 2 BMLs, which encompass both the central region and either the medial or the lateral compartment) and determine their relationship to cartilage loss and ACL tears.
Methods
Magnetic resonance imaging (MRI) of the knee was performed at baseline and at followup (15 and/or 30 months) in 258 subjects with symptomatic osteoarthritis (OA). At baseline, we assessed ACL tears and central BMLs located at or between the tibial spines or adjacent to the femoral notch. Cartilage loss was present if the score in any region of the tibiofemoral joint increased by ≥1 units at the last available followup, using a modified Whole-Organ MRI Score. We used logistic regression adjusted for alignment, body mass index, Kellgren/Lawrence score, sex, and age.
Results
One hundred thirty-nine knees (53.8%) had central BMLs, of which 129 had type 1 BMLs (96 abutted the ACL and had no coexistent type 2 features) and 25 had type 2 BMLs (often overlapped with type 1). Type 1 lesions were associated with ACL tears (odds ratio [OR] 5.9, 95% confidence interval [95% CI] 2.2–16.2) but not with cartilage loss (OR 1.6, 95% CI 0.8–3.1), while medial type 2 BMLs were related to medial cartilage loss (OR 6.1, 95% CI 1.0–35.2).
Conclusion
Central BMLs that abutted the ACL were highly prevalent and strongly related to ACL pathology, suggesting a role of enthesopathy in OA. Only BMLs with medial extension were related to ipsilateral cartilage loss.
Bone marrow lesions (BMLs) are areas of abnormal magnetic resonance imaging (MRI) signals that histologically represent necrosis, fibrosis, bleeding, and edema (1). The focus of research until now has been BMLs of the lateral or medial tibiofemoral compartment (2–6), which occur in the cortical subchondral region and strongly predict subsequent overlying cartilage loss (7). Their presence has been related to malalignment (8) and excess loading (9). Osteoarthritic (OA) knees with medial BMLs were more likely to have varus alignment, whereas lateral BMLs were more common in the presence of valgus alignment (8). Moreover, these lesions have also been associated with concomitant increased local bone density, which suggests that they may be secondary to long-term excess loading (9).
However, central BMLs (between the tibial spines, at the spines themselves, and at the femoral notch), localized in an area uncovered by cartilage and not directly involved in weight bearing, have not been assessed. One reason for this neglect is that the Whole-Organ MRI Score (WORMS), the most common grading system used when reading MRIs (10), does not grade lesions in the femoral notch. Unlike BMLs in the weight-bearing compartments, many central BMLs do not abut cortical bone but surround the insertions of the anterior cruciate ligament (ACL) or the posterior cruciate ligament (PCL), suggesting a different etiology related to cruciate damage and perhaps enthesopathy. On the other hand, some central lesions may represent extensions of an adjacent medial or lateral BML and, like these lesions, reflect the bone response to increased loading.
We suggest the existence of 2 nonexclusive types of central BMLs: type 1 lesions, which are anatomically related to ACL/PCL insertion, and type 2 lesions, which encompass the central compartment and either the medial or the lateral compartment. We hypothesized that type 1 lesions will not increase the risk of cartilage loss, but should be associated with ACL tears (because PCL tears are extremely infrequent, we focused only on ACL tears). We further hypothesized that type 2 lesions are extensions of lesions in either the medial or the lateral compartment and, like these, should increase the risk of cartilage loss in the appropriate compartment.
The purposes of this study were to describe the overall prevalence of central BMLs, to assess the frequency of the 2 types of central lesions described above, and to address their association with cartilage loss and ACL tears, testing our hypotheses.
Subjects and Methods
Study participants
The Boston Osteoarthritis of the Knee Study (BOKS) was a 30-month natural history study of symptomatic knee OA conducted from 1997 to 2001. Participants were recruited from 2 prospective studies of quality of life among veterans, 1 in men (≥45 years of age) and 1 in women (≥50 years of age); from clinics at the Veterans Administration (VA) Boston Healthcare System in Boston, MA; and from advertisements in local newspapers (2). Eligible participants must have answered yes to the following 2 questions: “Do you have pain, aching or stiffness in one or both knees on most days?” and “Has a doctor ever told you that you have knee arthritis?” and they must have screened negative in a questionnaire to rule out other forms of arthritis. They also had to have an osteophyte found on radiographs of their symptomatic knee and must have been able to walk, with or without the aid of a cane. Thus, all participants met the American College of Rheumatology criteria for OA (11). Subjects were also asked about any previous knee injury that required the use of crutches or a cane and about previous knee surgery (meniscectomy, ligament repair, arthroscopy, other).
The study included a baseline examination and followup examinations at 15 and 30 months. An MRI of the more symptomatic knee was obtained at baseline and at followup examinations, if there was no contraindication. At the first followup visit, long-limb films were obtained using a 14-inch × 51-inch cassette to measure mechanical alignment, using methods previously described (7).
Of the 317 members in the BOKS cohort, in this study we focused on 258 persons with baseline and followup MRIs because we wanted to evaluate cartilage loss on MRIs subsequent to BMLs. The institutional review boards of Boston Medical Center and the VA Boston Healthcare System approved the protocol.
MRI protocols and definitions
MRIs were obtained with a Signa 1.5T system (GE Medical Systems, Milwaukee, WI) using a phased array knee coil. An anchoring device for the ankle and knee was used to ensure uniformity of positioning among patients.
The imaging protocol included sagittal spin-echo proton density (PD) and T2-weighted images as well as T2-weighted, spin-echo coronal and axial, fat-suppressed, and PD images (time to recovery 2,200 msec, time to echo 20–80 msec, slice thickness 3 mm, interslice gap 1 mm, number of excitations 1, field of view 11–12 cm, matrix 256 × 192 pixels).
Definition of central BML
A central BML was defined as a poorly marginated area (without a defined rim or homogeneous fluid appearance) within the trabecular bone, with increased signal in the T2-weighted fat sequence localized in the coronal view at or between the spinous tibial plateaus or adjacent to the femoral notch (area not covered by cartilage). A musculoskeletal radiologist (DG) performed this part of the reading.
Type 1 lesions, which are anatomically related to ACL/PCL insertion, occurred when the lesion touched the cruciate ligament insertion(s) in the coronal and/or the sagittal view. Type 2 lesions, which are lesions that encompass the central and either the medial or the lateral compartment, occurred when the lesion's volume was in the central region but also extended into the medial or lateral compartment.
The characterization of central BMLs was performed by consensus between a trained reader (GH-M) and a musculoskeletal radiologist (AG) in 50% of the sample. In the rest of the MRIs, the trained reader performed the characterization. A random subset of images (n = 18) was read for interobserver reproducibility (88% agreement).
Cartilage loss
Using the WORMS system, cartilage signal and morphology on MRIs were scored in 5 regions of the tibiofemoral joint (central and posterior femur, and anterior, central, and posterior tibia) each for the medial and lateral compartments in the T2-weighted, fat-suppressed images. These features were graded on a scale from 0 to 6, where 0 = normal thickness and signal, 1 = normal thickness but increased signal, 2 = solitary partial thickness focal defect, 3 = multiple areas of partial thickness with areas of preserved thickness, 4 = diffuse partial-thickness loss, 5 = areas of full-thickness loss with areas of partial-thickness loss, and 6 = diffuse full-thickness loss (>75% of the region) (10). As previously described (12), we collapsed the original WORMS cartilage scores to a 0–4 scale, where the original WORMS scores of 0 and 1 were collapsed to 0, the original scores of 2 and 3 were collapsed to 1, and the original scores of 4, 5, and 6 were considered as 2, 3, and 4. Cartilage loss (in the medial or the lateral compartment) was defined as an increase in score of ≥1 units in any of the regions at followup. A total of 3 trained readers scored all MRIs as previously described (12), and the images from a majority of subjects (86%) with longitudinal MRIs were read by both a musculoskeletal radiologist and a musculoskeletal researcher, reading together. The intraclass correlation coefficient for agreement on cartilage readings ranged from 0.72 to 0.97 (12).
ACL tears
The presence of an ACL tear at baseline was determined using sagittal and coronal views and scored on a 0–2 scale, with 0 = normal, 1 = partial tear, and 2 = complete tear. A complete tear was defined as complete disruption of ACL fibers with ligament discontinuity, while a partial tear was defined as a partial interruption of the ACL fibers on at least 1 pulse sequence. All ACL tears were read by 1 board-certified musculoskeletal radiologist (AG) (weighted κ = 0.75).
Statistical analysis
Data were expressed as the mean ± SD. Baseline characteristics among the groups with and without central BMLs were compared using Student's t-tests and chi-square tests, when appropriate. For cartilage loss, we used 30-month followup data for all analyses unless they were not available, in which case 15-month data were used. Cartilage loss (medial or lateral separately) was analyzed as a dichotomous variable using logistic regression adjusted for alignment, body mass index (BMI), Kellgren/Lawrence (K/L) score (13), sex, and age to examine its association with each of the 2 types of central BML. The same model was used to test for the association of type of central BML and ACL tears (partial and complete ACL tears considered together). Statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC). P values less than 0.05 (2-tailed) were considered significant.
Results
Central BMLs
In the BOKS cohort, 317 participants underwent baseline knee MRI in their more symptomatic knee (1 knee per person), of whom 277 underwent followup MRI (either at 15 months or at 30 months). Then, we excluded 19 subjects who had unreadable MRIs and/or had any missing information regarding central BMLs. Ultimately, we focused on 258 knees (30 with only 15 months of followup and 228 with 30 months). Subjects were predominantly male (57.4%), and the mean ± SD age was 66.6 ± 9.2 years. The characteristics of the subjects, according to the presence or absence of central BMLs, are shown in Table 1.
Table 1.
Characteristics of the participants according to the presence or absence of central BMLs*
| All knees (n = 258) | Knees with central BMLs (n = 139) | Knees without central BMLs (n = 119) | |
|---|---|---|---|
| Age, mean ± SD years | 66.6 ± 9.2 | 67.5 ± 9.8 | 65.7 ± 8.6 |
| Women | 110 (42.6) | 61 (43.9) | 49 (41.2) |
| BMI, mean ± SD kg/m2 | 31.4 ± 5.7 | 31.4 ± 5.5 | 31.5 ± 6.1 |
| K/L score† | |||
| 0 | 17 (6.7) | 4 (2.9) | 13 (11.3) |
| 1 | 42 (16.7) | 13 (9.5) | 29 (25.2) |
| 2 | 58 (23.0) | 30 (21.9) | 28 (24.4) |
| 3 | 91 (36.1) | 55 (40.2) | 36 (31.3) |
| 4 | 44 (17.5) | 35 (25.5) | 9 (7.8) |
| Knee injury‡ | 63 (29.5) | 38 (32.7) | 25 (25.7) |
| Knee surgery§ | |||
| Meniscectomy | 13 (5.0) | 11 (7.9) | 2 (1.7) |
| Ligament repair | 13 (5.0) | 10 (7.1) | 3 (2.5) |
| Arthroscopy | 49 (19.1) | 27 (19.4) | 22 (18.8) |
| Other | 2 (0.7) | 2 (1.4) | 0 (0.0) |
| Any surgery | 68 (26.5) | 43 (30.9) | 25 (21.3) |
Except where indicated otherwise, values are the number (%). BMI = body mass index; K/L = Kellgren/Lawrence.
For knees with and knees without central bone marrow lesions (BMLs), n = 137 and n = 115, respectively. P < 0.0001, knees with versus knees without BMLs.
For knees with and knees without central BMLs, n = 116 and n = 97, respectively.
For knees without central BMLs, n = 117. Some patients underwent more than 1 surgical procedure.
Among all knees, 139 (53.8%) had central BMLs at the baseline evaluation: 12 in the femoral region (8.6%), 99 in the tibial region (71.2%), and 28 in both (20.1%). Knees with central BMLs had more advanced radiographic OA (Table 1).
Type 1 lesions
Of the 139 knees with central BMLs, 129 had type 1 lesions (92.8%). Type 1 central BMLs could abut the ACL, PCL, or both. Fifty-one of 129 knees (39.5%) had type 1 lesions involving the PCL. The majority of type 1 lesions (115 of 129 [89.1%]) abutted the ACL insertion (Figure 1A). Ninety-six of 115 knees (83.4%) had type 1 lesions around the ACL that did not additionally have type 2 features. We used this group of knees, with “pure” type 1 lesions, to address their association with cartilage loss and ACL tears (Figure 2).
Figure 1.
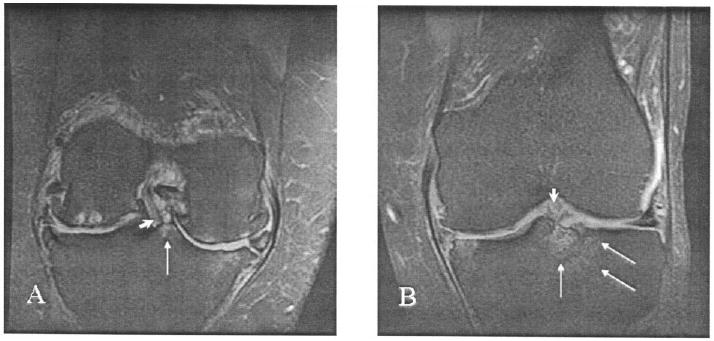
Two types of central bone marrow lesion, as shown in coronal, fat-suppressed, T2-weighted magnetic resonance images. A, An anterior cruciate ligament (ACL) insertion (arrowhead) bordered by a grade 1 posterior tibial type 1 lesion (arrow). Bicompartmental tibiofemoral cartilage loss and osteophytes, subchondral bone cysts in the lateral femoral condyle, bone marrow edema in the medial tibia and femur, and partial maceration of both menisci are also shown. B, An ACL insertion (arrowhead) bordered by a grade 2 anterior tibial type 2 lesion with lateral extension (arrows). Mild cartilage thickening and small osteophytes in the medial tibial plateau and femoral condyle, bone marrow edema of the medial tibial plateau, and partial maceration and subluxation of the medial meniscus are also shown.
Figure 2.
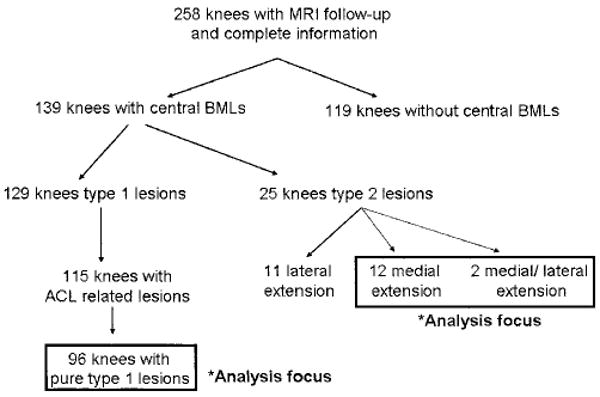
Flow diagram of the studied knees. MRI = magnetic resonance imaging; BMLs = bone marrow lesions; ACL = anterior cruciate ligament.
Type 2 lesions
Twenty-five of 139 knees (17.9%) were identified as having type 2 central BMLs: 12 with medial extension, 11 with lateral extension, and 2 with both medial and lateral compartment extension. The majority of knees (22 of 25) with type 2 lesions also had coexistent type 1 lesions (Figure 1B). To determine the association between ipsilateral medial or lateral cartilage loss and ACL tears, we considered knees with type 2 lesions with medial (n = 14) and lateral (n = 13) extension separately.
Cartilage loss and central BMLs
First, 11 of the 258 knees were excluded from the cartilage analysis because subject movement at followup limited the reading of cartilage morphology. During followup, 114 knees (46.1%) showed a loss of cartilage in ≥1 regions of the medial tibiofemoral joint. Because only 55 knees (22.2%) had a loss of cartilage at the lateral compartment and also because of the small number of knees with type 2 lesions with lateral extension, we decided not to perform any further analysis of this compartment. Thus, the analysis of the type 2 lesion group included only those knees with extension to the medial compartment (Figure 2).
We found that in the subset of knees with type 1 lesions abutting the ACL, this was not associated with medial cartilage loss (adjusted odds ratio [OR] 1.6, 95% confidence interval [95% CI] 0.8–3.1). Conversely, in the subset of knees with type 2 lesions with medial extension, this was strongly associated with medial cartilage loss (adjusted OR 6.1, 95% CI 1.0–35.2) (Table 2).
Table 2.
Types of central BMLs and their association with medial cartilage loss*
| Medial cartilage loss | ||
|---|---|---|
| No. (%) | Adjusted OR (95% CI) | |
| Knees with no central BMLs | 45/115 (39.1) | 1.0 (referent) |
| Knees with type 1 BMLs only (subset around ACL) | 48/93 (51.6) | 1.6 (0.8–3.1) |
| Knees with type 2 BMLs with medial extension† | 8/11 (72.7) | 6.1 (1.0–35.2) |
BMLs = bone marrow lesions; OR = odds ratio; 95% CI = 95% confidence interval; ACL = anterior cruciate ligament.
Knees with medial and medial-lateral extension.
ACL tears and central BMLs
We found that 59 of the 139 knees with overall central BMLs (42.4%) had ACL tears, whereas only 9 of 119 of knees without central lesions (7.6%) had tears. ACL tears were associated both with the subset of type 1 lesions that abutted the ACL and with type 2 lesions with medial extension (Table 3). Because, as noted above, almost all the knees in the type 2 category had coexistent type 1 lesions, it was not possible to analyze “pure” type 2 lesions. The adjusted OR for an ACL tear among the subset of knees with type 1 lesions was 5.9 (95% CI 2.2–16.2), and for knees with type 2 lesions with medial extension it was 8.2 (95% CI 1.5–45.3).
Table 3.
Types of central BMLs and their association with ACL tears*
| ACL tear | ||
|---|---|---|
| No. (%) | Adjusted OR (95% CI) | |
| Knees with no central BMLs | 9/119 (7.6) | 1.0 (referent) |
| Knees with type 1 BMLs only (subset around ACL) | 41/96 (42.7) | 5.9 (2.2–16.2) |
| Knees with type 2 BMLs with medial extension† | 8/14 (57.1) | 8.2 (1.5–45.3) |
See Table 2 for definitions.
Knees with medial and medial-lateral extension.
Discussion
Our results showed that central BMLs were highly prevalent and often localized to ACL or PCL insertions. We hypothesized that there are 2 nonexclusive types of lesions, those related anatomically to ACL or PCL insertion (type 1), and those encompassing the central compartment and either the medial or the lateral tibiofemoral compartment (type 2). We found that type 1 lesions that abutted the ACL were strongly related to ACL tears, but only type 2 lesions with extension to the medial compartment were associated with subsequent medial cartilage loss.
Studies to date have focused on the description of BMLs in the medial and lateral compartments (2–9). Lesions in the center of the knee have not been described thus far. In the present study, central BMLs were found in at least half of the knees studied. According to our classification, type 1 lesions were the more prevalent category, mainly involving the ACL insertion and localized at the tibial region. In the literature, other subchondral changes related to cruciate ligament attachments, such as insertional cysts, have been reported, but less frequently (14).
The prevalence of complete ACL tears in this cohort has been reported previously (22.8%) (15). We also have reported that ACL tears were not associated with sex, age, or BMI, but were related to severe radiographic OA and medial joint space narrowing (a reflection of damage to hyaline articular cartilage as well as meniscus alterations). In unpublished observations on the same cohort, it was shown that complete ACL tears increased the risk of cartilage loss, but this finding was totally mediated by concurrent meniscal pathology (Amin S: personal communication).
In the literature, other factors, such as the notch width and depth and the presence of notch osteophytes, have also been linked with ACL tears (16,17). ACL tears in our cohort were not related to a recalled knee injury (15); thus, these tears may be a consequence of ACL attrition. This study shows that ACL tears (complete and partial) are strongly associated with the presence of central BMLs.
In light of the surprisingly high prevalence of BMLs that abut the ACL and PCL, we suggest that enthesopathy may be an element in the etiology of these lesions. We noted that, although there was an association of type 1 lesions (that abut the ACL insertion) with ACL tears, most of the knees with these lesions had no ACL or PCL tears (57.6% and 99.2%, respectively). This may suggest that these BMLs antedate pathology in the ligaments, suggesting possible disease-related pathology at ligament insertion sites in OA. The etiology of this pathology needs study; it could be a primary disease process or could be a consequence of tensile stress on these ligaments in knees with OA (17).
Previous data support the possibility that ligament pathology may play a role in OA (18,19). Collateral ligament abnormalities, such as disruption, thickening, indistinct appearance, and increased signal, have been demonstrated by MRI of proximal and distal interphalangeal joints of OA hands, even in joints where cartilage was partially preserved (18). Further, as with our findings in the knee, there was bone edema at ligament insertion sites; in these patients, BMLs were localized on the proximal side of the joints, directly adjacent to sites where the collateral ligaments exert pressure on the proximal phalanx.
Another important finding of our study was the different behavior of the types of central BMLs regarding their relationship to cartilage. BMLs in the medial and lateral compartments predispose to joint space narrowing and cartilage loss (7,8). In our cohort, the prevalence of BMLs (grade ≥2) in the lateral and medial tibiofemoral joint compartments was 51% for the knees with central BMLs and 33% for the group without central BMLs. The pathologic process in the subarticular marrow of these compartments has been described as a response to malalignment (8) and overloading (9).
Herein, we found that only type 2 central BMLs were associated with cartilage loss, even if they coexisted with type 1 features; conversely, “pure” type 1 lesions that abutted the ACL were not associated with cartilage loss. There were no demographic differences among subjects identified as having type 1 and type 2 lesions in terms of age (mean ± SD 67.4 ± 9.4 years versus 62.5 ± 10.6 years; P = 0.07), BMI (31.3 ± 5.6 kg/m2 versus 31.0 ± 5.1 kg/m2; P = 0.84), and K/L score (grade 2 20.2% versus 14.2%, grade 3 42.5% versus 21.4%, and grade 4 24.4% versus 57.1%; P = 0.09).
Type 2 lesions may represent a merging of 2 independent BMLs (type 1 central lesions and lesions in an adjacent compartment) or may just be a subset of central lesions related to worsening disease. However, it seems that central lesions with compartment extension are more closely related to BMLs in the weight-bearing compartments.
Our study has the following limitations. First, it only includes patients with symptomatic knee OA. BMLs are linked to pain in some people (2–6), so it is possible that we had a selection bias resulting in an overestimation of the prevalence of central lesions in OA. Second, because we had few cases with lateral cartilage loss, we were unable to evaluate its association with ipsilateral BMLs. Nevertheless, we were able to study patients with cartilage loss that localized to the medial compartment. Third, because of the cross-sectional design to address the association between ACL tears and central BMLs, we cannot make longitudinal or causal inferences about the relationship between these central lesions and ACL pathology or OA. However, we found a strong relationship between central lesions that abut the ACL and ACL pathology, and our results should encourage a consideration of enthesopathy as a participant in the OA disease process. Finally, we used BMLs at baseline as predictors of cartilage loss at 15 or 30 months, which raises the question of how constant these lesions are. It has been shown in the same cohort (7) that BMLs of the medial and lateral compartments are unlikely to resolve and often get larger over time. This may also apply to lesions in the center of the knee, but this has not been studied.
In summary, the major finding of our study was that central BMLs that abut the ACL are frequently found in symptomatic OA knees and are strongly related to ACL pathology. However, when part of their volume also covers the medial compartment, central BMLs can behave like BMLs in the medial weight-bearing compartment, with a strong association with ipsilateral cartilage loss.
Acknowledgments
Supported by grants from the NIH (AR-47785), the Arthritis Foundation (Osteoarthritis Biomarkers Grant), and the Bayer Corporation.
Footnotes
Author Contributions: Dr. Felson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Felson.
Acquisition of data. Hernández-Molina, Guermazi, Gale, Goggins, Amin, Felson.
Analysis and interpretation of data. Hernández-Molina, Guermazi, Niu, Gale, Amin, Felson.
Manuscript preparation. Hernández-Molina, Guermazi, Amin, Felson.
Statistical analysis. Niu.
References
- 1.Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–40. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 3.Sowers MF, Hayes C, Jamadar D, Capul D, Lachance L, Jannausch M, et al. Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and X-ray-defined knee osteoarthritis. Osteoarthritis Cartilage. 2003;11:387–93. doi: 10.1016/s1063-4584(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 4.Zhai G, Blizzard L, Srikanth V, Ding C, Cooley H, Cicuttini F, et al. Correlates of knee pain in older adults: Tasmanian older adult cohort study. Arthritis Rheum. 2006;55:264–71. doi: 10.1002/art.21835. [DOI] [PubMed] [Google Scholar]
- 5.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14:1033–40. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Felson DT, Niu J, Guermazi A, Roemer F, Lewis CE, Torner J, et al. The development of knee pain correlates with enlarging bone marrow lesions on MRI [abstract] Arthritis Rheum. 2006;(54) 9:S533. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 7.Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, LaValley MP, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthrits. Arthritis Rheum. 2006;54:1529–35. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 9.Lo GH, Hunter DJ, Zhang Y, McLennan CE, LaValley MP, Kiel DP, et al. Bone marrow lesions in the knee are associated with increased local bone density. Arthritis Rheum. 2005;52:2814–21. doi: 10.1002/art.21290. [DOI] [PubMed] [Google Scholar]
- 10.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Altman R, Ach E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 12.Amin S, LaValley MP, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, et al. The relation between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52:3152–9. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 13.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaren DB, Buckwalter KA, Vahey TN. The prevalence and significance of cyst-like changes at the cruciate ligament attachments in the knee. Skeletal Radiol. 1992;21:365–9. doi: 10.1007/BF00241814. [DOI] [PubMed] [Google Scholar]
- 15.Hill CL, Seo GS, Gale D, Totterman S, Gale ME, Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52:794–9. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 16.Lee GC, Cushner FD, Vigoritta V, Scuderi GR, Insall JN, Scott WN. Evaluation of the anterior cruciate ligament integrity and degenerative arthritic patterns in patients undergoing total knee arthroplasty. J Arthroplasty. 2005;20:59–65. doi: 10.1016/j.arth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Wada M, Tatsuo H, Baba H, Asamoto K, Nojyo Y. Rheumatology (Oxford) Vol. 38. 1999. Femoral intercondylar notch measurements in osteoarthritic knees; pp. 554–8. [DOI] [PubMed] [Google Scholar]
- 18.Tan AL, Grainger AJ, Tanner SF, Shelley DM, Pease C, Emery P, et al. High-resolution magnetic resonance imaging for the assessment of hand osteoarthritis. Arthritis Rheum. 2005;52:2355–65. doi: 10.1002/art.21210. [DOI] [PubMed] [Google Scholar]
- 19.Quasnichka HL, Anderson-MacKenzie JM, Tarlton JF, Sims TJ, Billingham ME, Bailey AJ. Cruciate ligament laxity and femoral intercondylar notch narrowing in early-stage knee osteoarthritis. Arthritis Rheum. 2005;52:3100–9. doi: 10.1002/art.21340. [DOI] [PubMed] [Google Scholar]


