Table 1.
Development of Organo-SOMO Aldehyde α-Arylation.
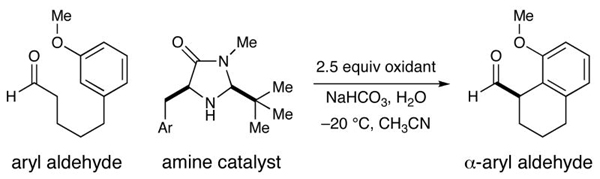 | ||||
|---|---|---|---|---|
| entry | oxidant | catalyst (Ar) % | yielda | % eeb |
| 1 | CAN | Ph, 2 (30 mol %) | 63 | 84 |
| 2 | [Fe(phen)3]·(PF6)3 | Ph, 2 (30 mol %) | 60 | 92 |
| 3c | [Fe(phen)3]· PF6)3 | Ph, 2 (30 mol %) | 85 | 92 |
| 4c | [Fe(phen)3]·(PF6)3 | Ph, 2 (20 mol %) | 61 | 90 |
| 5c | [Fe(phen)3]·(PF6)3 | 1-Naphth, 5 (20 mol %) | 70 | 97 |
| 6c,d | [Fe(phen)3]·(PF6)3 | 1-Naphth, 5 (20 mol %) | 80 | 98 |
Isolated yields of the corresponding alcohol.
Enantiomeric excess determined by chiral HPLC.
30 mol % pivalic acid.
50 mol % H2O.
