Abstract
We conducted a clinical trial of peptide PSA:154–163(155L) vaccination in HLA-A2 patients with detectable and rising serum PSA after radical prostatectomy for prostate cancer (Clinicaltrials.gov identifier NCT00109811). The trial was a single dose-level, Phase II pilot trial of 1 mg of PSA154–163(155L) emulsified with adjuvant (Montanide ISA-51). The primary endpoint was determination of immunogenicity of the vaccine; secondary outcomes were determination of toxicity and effect on serum PSA. The vaccine was given subcutaneously 7 times on weeks 0, 2, 4, 6, 10, 14, and 18. Peptide-specific CD8 T-cell responses in the peripheral blood mononuclear cells (PBMC) of patients were measured by IFN-γ ELISPOT assay. CD8 T-cell cultures were also established by in vitro stimulation with the peptide presented by autologous dendritic cells. Five patients were enrolled and completed all vaccinations. No IFN-γ response to PSA:154–163(155L) was detected in unfractioned PBMC in any patient either before or after vaccination. Three of five patients demonstrated strong IFN-γ responses to PSA:154–163(155L) as well as native PSA:154–163 peptides in CD8 T-cell cultures derived from post-vaccination PBMC. However, peptide-specific T cells failed to recognize HLA-A2 targets expressing endogenous PSA. There were no significant changes in serum PSA level in any subject. No serious adverse events were observed. PSA154–163(155L) is not an effective immunogen when given with Montanide ISA-51. The PSA154–163 peptide is poorly processed from endogenous PSA and therefore represents a cryptic epitope of PSA in HLA-A2 antigen-presenting cells.
Keywords: prostate cancer, clinical trial, PSA, peptide vaccine, immunological monitoring
Introduction
Adenocarcinoma of the prostate is one of the most common malignancies of men in the United States. While radical prostatectomy and radiation therapy are effective treatments for prostate cancer localized to the gland, it remains arguable whether such definitive local therapy extends patient survival or not, especially in light of the side effects of treatment.1–3 With current clinical staging procedures it is possible to select men with clinically localized prostate cancer who are likely to do well after treatment for their localized disease. However, 30,000 men die of prostate cancer annually in the U.S. and they die of metastatic disease. Effective systemic therapy for advanced prostate cancer is limited and non-curative. Given the promise of immunotherapy in other malignancies, the development of effective systemic immunotherapies able to extend the survival of men with the metastatic prostate cancer would be a significant advance.
Prostate specific antigen (PSA) is a major secretory product of the prostatic epithelium, both benign and malignant. PSA appears in the serum in normal men but can be elevated by benign prostatic hypertrophy as well as cancer of the prostate. PSA has been found to be a highly reliable tumor marker for prostatic cancer.4 If the entire prostate gland is removed then PSA should fall to undetectable or female levels.5 The presence of PSA in the serum after radical prostatectomy suggests that prostatic epithelial cells, either benign or malignant, remain in the patient at some location. There is no standard therapy option for patients with detectable PSA after radical prostatectomy although the use of postoperative radiation has been shown to increase the prostate cancer-specific survival in men with short PSA doubling times.6 The median interval between the development of detectable PSA following radical prostatectomy to the development of metastatic disease was 8 years in the series described by Pound et al.7 Hence, in such men there is a significant interval before progression during which patients could be treated with alternate therapies. Options for therapy are expectant management, hormonal ablation therapy and pelvic radiation therapy.
Human tumors contain antigens that can be specifically recognized by the immune system and this recognition can be exploited to be therapeutic.8–10 Peptide vaccination in different types of cancer has clearly demonstrated immunologic reactivity and some evidence of anti-tumor responses 11–21 but the overall effectiveness of peptide vaccination as a treatment for human cancer has been minimal.22 Toxicity has been low with mainly injection site reactions and reactions to adjuvant agents observed.
Many peptides derived from PSA have been shown to stimulate CTL responses in vitro and in vivo.23–27 The approach based on the use of PSA as an antigen for vaccination in prostate cancer has reached human clinical trials. Eder et al 28 in a phase I trial of recombinant vaccinia PSA (rV-PSA) with or without GM-CSF administered to 33 men with a rising PSA and hormone-naïve prostate cancer, demonstrated stable PSA levels in 14 of the 33 men. Immunologic studies demonstrated a specific T-cell response to the PSA-3 peptide. Similar results in a separate phase I study have been presented.29 PSA:154–163(155L) is part of the PSA-TRICOM vaccine, another recombinant vaccinia construct, which is being tested in current clinical trials in HLA-A2 patients with prostate cancer.30
We conducted a clinical trial of the peptide PSA:154–163(155L) (amino acid sequence VLSNDVCAQV representing residues 154–163 (155L) of mature secreted PSA sequence numbered without the 24 amino acid leader sequence) as a vaccination strategy for the treatment of prostate cancer in HLA-A2 patients with detectable and rising serum PSA after radical prostatectomy. The original peptide PSA:154–163 (VISNDVCAQV, designated as PSA-3) was described by Correale et al.24 PSA:154–163(155L) peptide is a modified agonist peptide (also designated PSA-3A, “A” for agonist) that was described by Terasawa et al.26 PSA:154–163(155L) is an agonist epitope of PSA-3, which represents the native PSA-3 peptide with substitution of leucine at position 155. The substitution increased the binding affinity of PSA:154–163(155L) to HLA-A2. T cell lines specific for PSA:154–163(155L) also recognized the native PSA-3 peptide PSA:154–163.26 The trial was a single dose-level, phase II pilot trial of 1 mg of PSA:154–163(155L) emulsified with adjuvant (Montanide ISA-51) given subcutaneously at two sites every other week four times, then every fourth week three times. The primary outcome was determination of immunologic laboratory endpoints, secondary outcomes were determination of toxicity and effect on serum PSA.
Materials and Methods
Study design and endpoints
The study was a pilot phase II study of PSA:154–163(155L) vaccination in men with detectable and rising PSA after radical prostatectomy where the primary endpoint was the determination of immunologic laboratory parameters. The purpose of the trial was to demonstrate that, at a minimum, the peptide is immunologically active and naturally processed and presented by antigen presenting cells. A response to peptide vaccination was defined as the detection of a PSA:154–163(155L)-specific CD8 T cell INFγ response above background in patients after vaccination in INFγ ELISPOT assay if no such response was observable before vaccination or a five fold increase in the frequency of such cells after vaccination if such cells were present before vaccination. The only tumor parameter followed on the study was serum PSA. A decline in serum PSA of at least 50% compared to baseline, confirmed by a second PSA value 4 weeks later, was scored as a response as a secondary endpoint. The other secondary endpoint was the determination of toxicity.
Sample size calculation
The primary endpoint of this study is the immunologic response rate of PSA:154–163(155L) vaccination in recurrent prostate cancer patients. We wished to test the null hypothesis of (undesirable) response rate ≤ 5% versus the alternative hypothesis of (promising) response rate ≥ 20%. Simon’s two-stage minimax design 31 was used to differentiate between these two response rates. Eighteen (18) qualified patients were planned in the first stage. At the end of the first stage, if no patients responded to the vaccination, the accrual would be stopped and the conclusion drawn that the vaccination was not worthy of further study. If at least one patient responds to the vaccination, a second group of 14 qualified patients would be enrolled into the second stage of this study. If, at the end of the second stage, 3 or fewer of the total of 32 patients had responded, the vaccination would be deemed not worthy of any further investigation in this study population. However, if 4 or more patients responded, the vaccination would be recommended for further study. This design has a statistical power of 90% with a type I error (false positive) rate at 0.07. The procedure gives a 40% chance of terminating enrollment early at the end of the first stage and an average sample size of 26 patients if the true response rate is as low as 5%.
Patients
The study was approved by the University of Maryland Institutional Review Board and informed consent to participate in the study was obtained from each subject. All patients were recruited from the practices of medical oncology and urology at the University of Maryland and the VA Maryland Health care system. Patients enrolled in the study had histologically proven adenocarcinoma of the prostate and had undergone radical prostatectomy at least 3 months before the first vaccination with no prior radiation therapy to the prostate and no hormonal therapy. Patients had no evidence of osseous metastases and no evidence of local recurrence by digital rectal examination. Serum PSA was ≥ 0.6 ng/ml and rising by any amount on at least two measurements separated by at least 3 months. Patients with metastatic prostate cancer, patients having previous chemotherapy, and patients with any form of primary or secondary immune deficiency were excluded from the trial. All patients were counseled by the investigators before inclusion and signed the informed consent document prior to enrollment. The trial was monitored by the University of Maryland Greenebaum Cancer Center Data Safety and Monitoring Board and by the Cancer Therapy Evaluation Program, National Cancer Institute.
HLA typing
All patients included in the study were HLA typed by flow cytometry for the presence of HLA-A2 allele using FITC-labeled anti-human HLA-A2 antibodies and corresponding isotype control (BD Biosciences, Franklin Lakes, NJ, USA). The samples were analyzed by FACSCalibur flow cytometer using CellQuest software (BD Biosciences). Subsequent high resolution HLA typing was performed using Micro SSP HLA Class I A Locus Specific typing kit (One Lambda, Canoga Park, CA, USA) according to the manufacturer’s instructions.
Vaccine preparation and administration
PSA:154–163(155L) (VLSNDVCAQV) peptide (NSC 722932) and Montanide® ISA 51 (NSC 675756) were supplied by the Pharmaceutical Management Branch, CTEP, DCTD, NCI. Each PSA:154–163(155L) peptide 1 mg vial was reconstituted with 1 mL of Sterile Water for Injection, USP. A volume of 1.1 mL of Montanide® ISA 51 was added to the reconstituted peptide vial and mixed with a vortex mixer for 12 minutes to form the water-in-oil emulsion. A total of 2 ml (1 mg) of PSA:154–163(155L) peptide emulsified with Montanide® ISA 51 was administered as two separate 1 ml subcutaneous injections in the anterior thigh deep subcutaneous tissue, 2 cm apart, on the same extremity on study weeks 0, 2, 4, 6, 10, 14, 20. Subsequent injections were rotated between proximal extremities.
Immunological monitoring
PBMC collection
Each patient underwent leukopheresis procedure twice – before the first vaccination (baseline) and within 2 weeks after the last peptide vaccination. PBMC were purified on Histopaque-1077 and cryopreserved in aliquots in the freezing media (10% DMSO, 90% human AB serum, Atlanta Biologicals Inc., Lawrenceville, GA). In addition, blood samples were collected before each vaccination. PBMC were purified by density gradient centrifugation on Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) using ACCUSPIN™ tubes (Sigma-Aldrich).
Peptides
Peptide PSA:154–163(155L) for the in vitro assays was supplied by the Pharmaceutical Management Branch, CTEP, DCTD, NCI. The native peptide PSA:154–163 without the leucine substitution was synthesized at the University of Maryland School of Medicine biopolymer/genomics core facility and purified to >90% by reversed-phase high-performance liquid chromatography. The molecular weights were confirmed by mass spectrometry. Influenza M1 matrix protein 58–65 peptide was synthesized at NeoMPS, Inc. (San Diego, CA).
ELISPOT assay
The human IFN-γ ELISPOT assay in vaccinated cancer patients has been performed as described with modifications.32 The IFN-γ ELISPOT assay was performed at the same time on cryopreserved PBMC purified from the leukopheresis product collected before the first vaccination (“baseline”) and after completion of all vaccinations for each patient. In addition, PBMC purified from fresh blood samples obtained at each study visit were tested for the presence of the PSA-3A peptide reactivity at different time points throughout the study (weeks 0, 2, 4, 6, 10, 14, 20). To measure IFN-γ production in fresh or cryopreserved/thawed unfractioned PBMC, 96-well MultiScreen Filter Plates for ELISPOT (Millipore MAIPS4510, Billerica, MA) were coated with anti-human IFN-γ capture antibody (human IFN-γ ELISPOT Pair, BD Biosciences) according to the manufacturer’s instructions. PBMC were plated at 2×105, 4×105 or 8×105 cells/well in quadruplicates in the presence or absence of PSA:154–163(155L) peptide (25 µg/ml) in Iscove's modified Dulbecco's medium (IMDM) with the following supplements: 2 mM L-glutamine, 0.1 mM MEM nonessential amino acids, 1 mM MEM sodium pyruvate, 100 units/ml penicillin, 100 µg/ml streptomycin, 0.05 mM 2-mercaptoethanol (all reagents from Invitrogen, Carlsbad, CA), 5% human AB serum (Atlanta Biologicals) referred to as complete IMDM. As a positive control, PBMC (2×104 cells/well) were incubated with Concanavalin A (5 µg/ml, Sigma-Aldrich). After 48 hr incubation at 37°C, 5%CO2, the assays were developed according to manufacturer’s instructions using biotinylated detecting Ab followed by Alkaline Phosphatase-conjugated Streptavidin (Invitrogen, Carlsbad, CA). The spots were developed with 100 µl/well of BCIP-NBT phosphatase substrate (KPL, Gaithersburg, MD). Spots were counted using the ImmunoSpot Imaging Analyzer system (Cellular Technology Ltd, Cleveland, OH, USA).
Development of CD8 T-cell lines specific for PSA:154–163(155L) peptide
PBMC were enriched for CD8 T cells by negative immunomagnetic selection using human CD4, CD14, CD19 Dynabeads (Invitrogen). The surface markers expressed by T-cell lines were determined by flow cytometry using anti-CD4-FITC/anti-CD8-PE, anti-TcRα/β-FITC, and IgG2a-FITC/IgG2a-PE mAbs (BD Biosciences and Coulter, Fullerton, CA). To generate dendritic cells (DC), CD14-enriched cells were prepared from PBMC by negative immunomagnetic selection using CD2 and CD19 Dynabeads (Invitrogen). CD14-enriched PBMC were differentiated in the presence of 100 ng/ml recombinant human interleukin (rhIL)-4 (PeproTech Inc., Rocky Hill, NJ) and 20 ng/ml recombinant human granulocyte macrophage colony–stimulating factor (rhGM-CSF, Sargramostim, Berlex, Seattle, WA) for 7 days. Half of the media was replaced on day 4 with the same volume containing with 100 ng/ml rhIL-4 and 20 ng/ml rhGM-CSF; maturation was induced by addition of 10 ng/ml recombinant human tumor necrosis factor α (R&D Systems, Inc., Minneapolis, MN) and 20 ng/ml recombinant human IFN-α (PBL InterferonSource, Piscataway, NJ). The phenotype of mature DC was assessed by staining with anti-CD14-FITC, anti-CD83-PE, anti-CD80-PE, anti-HLA-DR-PE, and anti-CD86-FITC (all mAbs from BD Biosciences). To develop peptide-specific CD8 T cell lines, CD8 T cell-enriched PBMC were cultured at 2 × 106 cells/well in the presence of irradiated (1,000 rad) mature autologous DC at 2 × 105 cells/well, and peptides at 25 µg/ml in the first round of peptide stimulation. For the second and third rounds of stimulation, CD8 T cells were added at 1 × 106 cells/well and DC were added at 1 × 105 cells/well. Cells were incubated in 2 ml/well in 24-well plates in complete IMDM media in the presence of 10 ng/ml recombinant human IL-7 (PeproTech Inc.) at 37°C in a humidified 5% CO2 atmosphere. Recombinant human IL-2 (Chiron Corp., Emeryville, CA) was added at 30 IU/ml on day 4. Media containing rhIL-2 and rhIL-7 was replaced twice a week. Cells were restimulated every 14 days.
CTL clones were derived from bulk CD8 T cell cultures by limiting dilution. Lymphocytes were plated in the U-bottom 96-well tissue culture plates at 10 or 100 cells per well in the presence of specific peptide, irradiated mature autologous DC (1 × 104 cells/well), and rhIL-7 (10 ng/ml). Allogeneic PBMC (a mixture of at least 3 HLA-mismatched donors) were irradiated at 5,000 rad and used as feeder cells (2 × 105 cells/well); rhIL-2 (30 U/mL) was added to the wells on day 3 after stimulation. The clones were restimulated every 10 days. After several cycles of stimulation clones were subsequently expanded by transferring into 24 well plates together with irradiated autologous DC (1 × 105 cells/well) and allogeneic PBMC (1 × 106 cells/well) in the presence of the specific peptide and rhIL-7 and rhIL-2.
To detect IFN-γ secretion in advanced cultures, CD8 T cells were harvested at resting stage, on day 14 after the exposure to antigen in a previous stimulation cycle and plated into ELISPOT plates at 20,000 cells/well in complete IMDM media. Effector cells were co-cultured with target cells in the presence or absence of the specific peptide; effector cells alone and target cells alone served as negative controls. The IFN-γ ELISPOT assay was performed as described above. In some experiments monoclonal antibodies were used to block peptide recognition. Monoclonal antibodies W6/32 (anti-HLA-A,B,C), L243 (anti-HLA-DR), and IA14 (IgG2a) for blocking studies were produced by culturing hybridoma cells (American Type Culture Collection, Manassas, VA), purified from conditioned culture supernatant using protein A/G columns (Pierce Biotechnology, Inc., Rockford, IL) and were added at 5 to 10 µg/ml.
Granzyme B secreting cells were detected using Human GrB ELISPOT Set (BD Biosciences) according to manufacturer’s instructions. Target cells were prepared as described below. Effector cells at different numbers (2-0.25×105 cells/well) were co-cultured with target cells (5×104 cells/well; effector-target ratio 4-0.5). Effector cells alone served as a negative control. After 24 hr incubation at 37°C, 5% CO2, the reaction was developed using biotinylated anti-human GrB detecting antibody and horse radish peroxidase(HRP)-conjugated Streptavidin according to manufacturer’s instructions. The spots were visualized with 3-amino-9-ethylcarbazole (AEC) Substrate (BD Biosciences) and counted using the ImmunoSpot Imaging Analyzer system.
Target cells
LNCaP and OVCAR3 cell lines (derived from HLA-A*0201+ donors) were purchased from ATCC (Manassas, VA, USA). HEK293-A2 cell line engineered to express HLAA* 0201 and HEK293-A2-PSA cell line engineered to express HLA-A*0201 and whole length PSA were generous gift from Dr. J. Schlom (The Center for Cancer Research, NIH, Bethesda, MD). OVCAR3-PSA cell line was produced by transfection of OVCAR3 cells with pSecTag2/Hygro/PSA plasmid (Invitrogen) using FuGene6 transfection reagent (Roche Applied Science, Indianapolis, IN, USA) and subsequent cloning. Cells transfected with pSecTag2/Hygro/A (Empty Vector - EV) plasmid served as a negative control. To upregulate HLA-A2 expression, OVCAR3-EV or OVCAR-PSA cells were incubated with rhIFN-γ (5 ng/ml, PBL InterferonSource) for 48 hr. HLA-A2 expression was detected by immunofluorescence staining. Concentration of secreted PSA by PSA-expressing cell lines was measured in cell culture supernatants by ELISA using a pair of capture and detecting mAbs (Fitzgerald Industries International, Concord, MA, USA). Detecting antibodies were biotinylated using Sulfo-NHS Biotinylation Kit (Pierce, Rockford, IL) according to manufacturer’s instructions. The assays were developed using the Alkaline phosphatase-conjugated Extravidin (Sigma) and pNPP substrate (Sigma). The amount of bound PSA was measured spectrophotometrically at 450/650 nm. For the cellular assays, OVCAR cells were pre-treated with IFN-γ for 48 hr and pre-incubated with the peptides overnight as indicated in the figure legends. Before the assay, the target cells were washed twice, then incubated for 2 hr in complete IMDM to remove traces of IFN-γ, trypsinized, washed two times with PBS, and plated at 5×104 cells/well. For IFN-γ ELISPOT, target cells were irradiated at 5,000 rad. For GrB ELISPOT targets were not irradiated.
For viral protein presentation experiments by OVCAR3 cells, Influenza virus strain A/Puerto Rico/8/34 (generously provided by Dr. D. Farber, University of Maryland, Baltimore, MD) was used to infect the cells. OVCAR3-EV and OVCAR3-PSA cells were grown to 90% confluence, washed twice with PBS, and viral stock was inoculated at multiplicity of infection (MOI)=2 in serum-free Dulbecco’s modified Eagle’s medium (DMEM) for 1 1/2 hr. Following incubation, DMEM containing 0.1% BSA was added, and the cells were incubated overnight. The media was removed, and DMEM containing 2 µg/ml L-1-tosylamido-2-phenylethyl chloromethyl ketone (TPCK) –treated trypsin (Sigma) and 2% BSA was added to the cells. The cells were incubated for additional 24 hr and used in the assays as described.
Statistics
The statistical significance of the results was determined using a two-tailed Student’s test. Statistical significance was set at p < 0.05.
Results
Patients
Thirty seven patients with recurrent prostate cancer were HLA typed before enrollment in the study. Of the 37 patients 17 were HLA-A2 positive by flow cytometry, five agreed to enroll in the study. The first patient was enrolled on 5/18/2005 and the last patient was enrolled on 1/24/2006 and was off study on 7/11/2006. The clinical characteristics of the patients are shown in Table 1. By high resolution typing three of the five patients were HLA-A*0201 (Pr143, Pr150, Pr159) and two patients were HLA-A*0205 (Pr142, Pr151).
Table 1.
Clinical characteristics of the patients enrolled in the study.
| SUBJECT | Age | Race | Years since RRP |
Pathologic stage |
Gleason score |
Post op radiation? |
|---|---|---|---|---|---|---|
| Pr142 | 59 | B | 11.5 | pT2bN0M0 | 5 | N |
| Pr143 | 65 | W | 11 | pT3aN0M0 | 6 | Y |
| Pr150 | 79 | W | 13.5 | pT3bN0M0 | 7 | Y |
| Pr151 | 59 | W | 9 | pT3aN0M0 | 6 | Y |
| Pr159 | 64 | W | 14.5 | pT3aN0M0 | 8 | Y |
The study was planned for accrual of 18 patients and then an interim analysis. However, as testing for laboratory endpoints proceeded it was not clear that the peptide was naturally processed and presented by HLA-A2 antigen presenting cells (see below), a characteristic that is essential for immunologic reactivity via class I-restricted recognition of tumor antigens. For this reason no new patients were recruited after first five patients were enrolled. Once it was clear that the peptide was poorly processed and presented the study was formally closed on 9/10/2007.
Clinical response
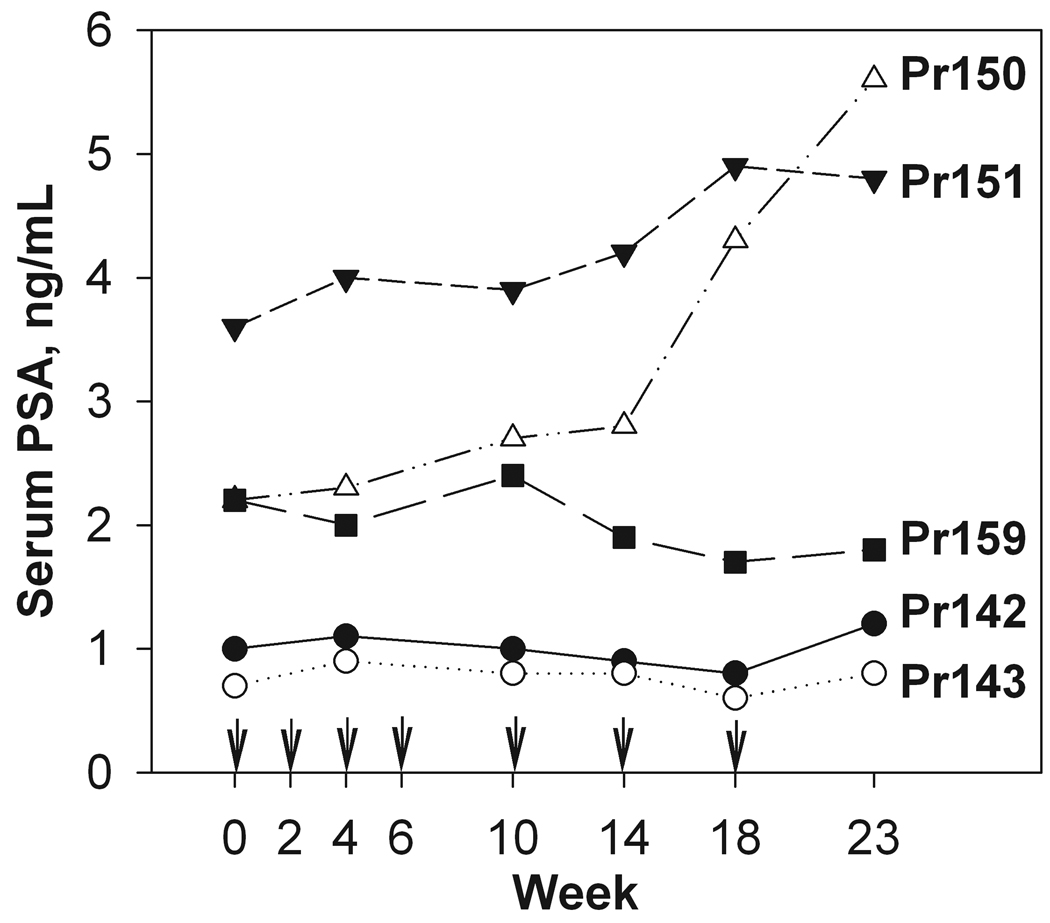
All five patients enrolled in the study completed all vaccinations. All five vaccinated patients tolerated the vaccinations well. The most common side effects of vaccination were a local injection reaction (redness, swelling at the site of the vaccination) and myalgia. A summary of all recorded toxicities for the study are shown in Table 2. All toxicities were grade 1 or 2; no serious adverse events were observed. A mild increase in the PSA level was detected in two of the patients (Pr150 and Pr151, Figure 1). There was no significant change in serum PSA in subjects Pr142, Pr143 and 159 (Figure 1).
Table 2. Summary of adverse events.
Data are number of reported events for all patients over the entire study duration *
| Adverse reaction | Number of events |
|---|---|
| Injection site reaction | 18 |
| Myalgia | 8 |
| Sinusitis or sinus congestion | 5 |
| Fatigue | 5 |
| Dizziness | 4 |
| Infection | 2 |
| Leukopenia | 2 |
| Edema | 2 |
| Low RBC count | 2 |
| Hypoglycemia | 1 |
| Nausea | 1 |
| Vomiting | 1 |
| Desquamation finger | 1 |
| Hematuria | 1 |
| Chest pain | 1 |
Grade 1 or 2 adverse events reported
FIGURE 1. Change in serum PSA over time.
Serum PSA levels were measured on the day of vaccination according to study protocol. Each line corresponds to individual patient. Arrows indicate time points of peptide vaccination.
Immunological monitoring
The IFN-γ ELISPOT assay on unfractioned cryopreserved PBMC was performed as the primary outcome of the study. PBMC were collected from all five study patients by automated leukopheresis at baseline (before the first injection) and within two weeks after the completion of all seven vaccinations with the peptide. In addition, blood samples were obtained at each study visit (weeks 0, 2, 4, 6, 10, 14, 20), and fresh PBMC were tested for the presence of the PSA-3A peptide reactivity. We were not able to detect PSA-3A peptide-specific response in either fresh or cryopreserved primary PBMC (tested at 2×105, 4×105 and 8×105 cells/well in replicates) at any time point during the course of vaccination (results are not shown).
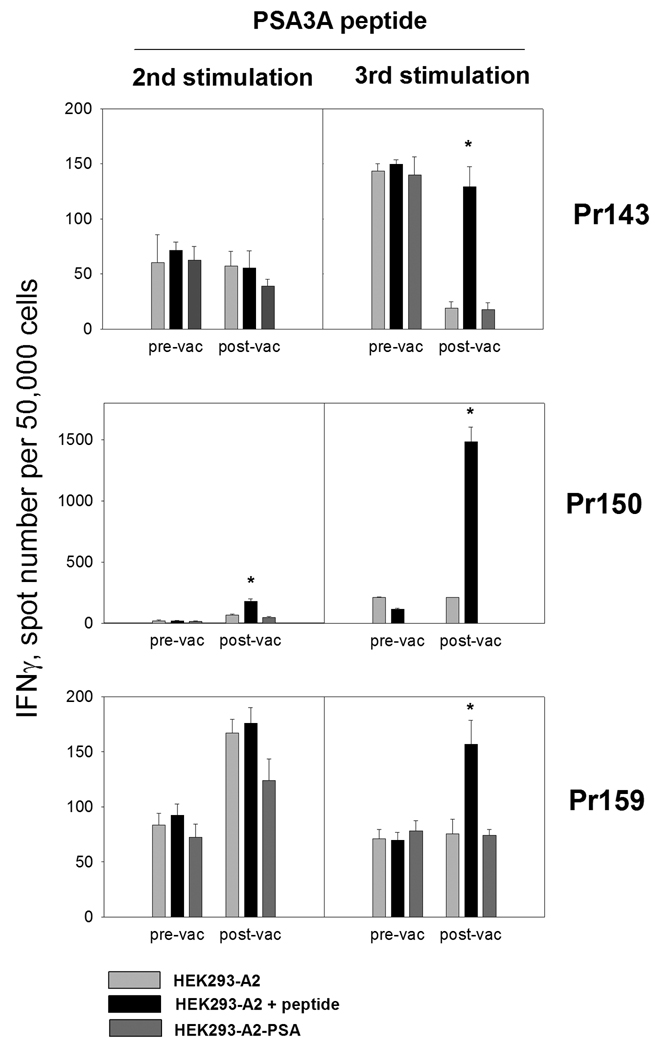
Because the ELISPOT assays were negative with unfractioned PBMC in all patients and in different conditions that we tested, we cultured CD8 T cells from PBMC from all patients before and upon completion of vaccinations. CD8 T cells were stimulated in vitro for one or two cycles with PSA:154–163(155L) peptide presented by autologous mature DC and tested for the reactivity with PSA:154–163(155L) peptide presented by HLA-A2 positive target cells. None of the five cultures derived from the baseline PBMC responded to the peptide. However, cultures developed from PBMC of three patients (Pr143, Pr150, Pr159) demonstrated strong PSA:154–163(155L)-specific IFN-γ production in cultured CD8 T cells after one or two rounds of stimulation in the post-vaccination cultures (Figure 2). In one of the patients (Pr150), high frequencies of peptide-specific IFN-γ producing cells were detected after only one round of antigenic stimulation with the PSA:154–163(155L) peptide, while two rounds of stimulation were necessary to detect presence of antigen-specific cells in the cultures of patients Pr143 and Pr159 by IFN-γ ELISPOT (Figure 2). In patient Pr142 we detected only a very low frequency of PSA:154–163(155L) peptide-specific CD8 T cells after one round of stimulation with the peptide (18 spots per 5×104 CD8 T cells, data not shown). The response was not sustained after additional in vitro stimulation. However, specificity of the response was confirmed by further cloning (Figure 5 and Figure 7B, clone Pr142 cl1). No response was detected in the cultures of patient Pr151 (data not shown). As a positive control, CD8 T cell cultures specific to HLA-A2 restricted influenza virus M1 matrix protein peptide 58–65 were also established from pre-vaccination and post-vaccination CD8 T-cell-enriched PBMC and tested in parallel with PSA:154–163(155L) peptide-specific cultures. Both baseline and post-vaccination CD8 T cell cultures responded vigorously to the influenza M1 peptide (data not shown).
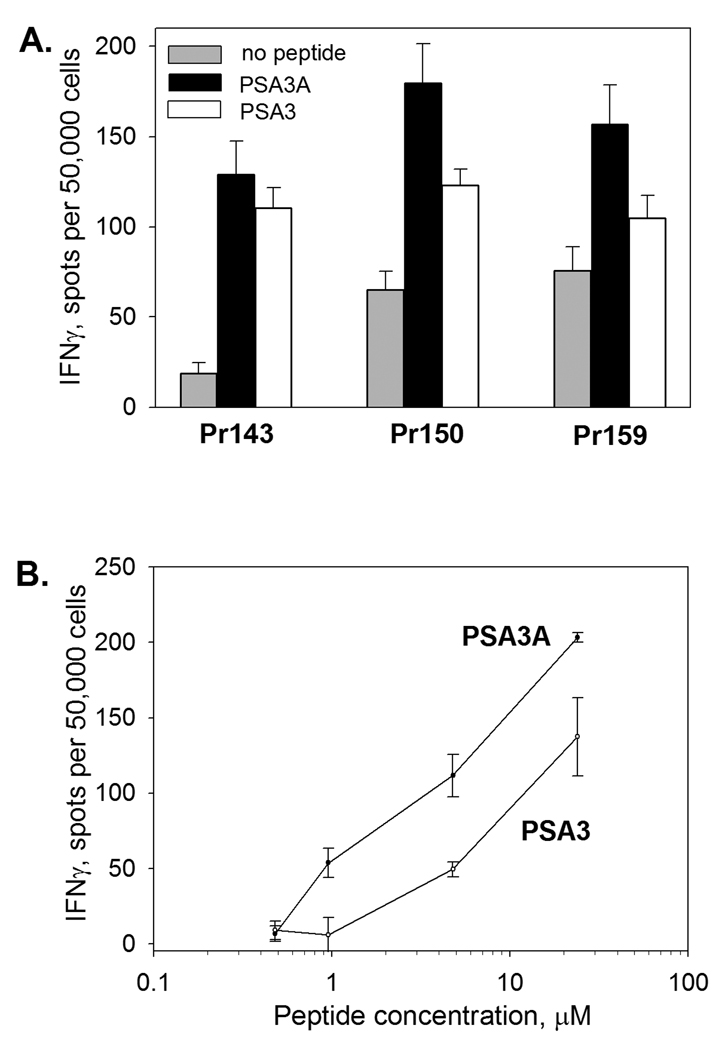
FIGURE 2. Peptide-specific CD8 T-cell response in prostate cancer patients before and after vaccination with peptide PSA154–165(155L) (peptide PSA3A) in Montanide ISA-51.
Cell lines were developed from CD8 T cell-enriched baseline (pre-vac) and post-vaccine (post-vac) PBMC by stimulation with PSA3A peptide presented by autologous DC. The cultures were tested for the reactivity with the specific peptide or PSA-expressing cell line after one or two cycles of in vitro stimulation by IFN-γ ELISPOT assay. T cells at 50,000 cells/well were co-cultured with irradiated HEK293-A2 cells in the presence (black bars) or absence (gray bars) of specific peptide for 48 hr or HEK293-A2-PSA cells (dark gray bars). Results for three responding patients are shown. The assays were developed as described in Materials and Methods. The data are mean ±SD of triplicate determinations. * p<0.0001 (2-sided t-test).
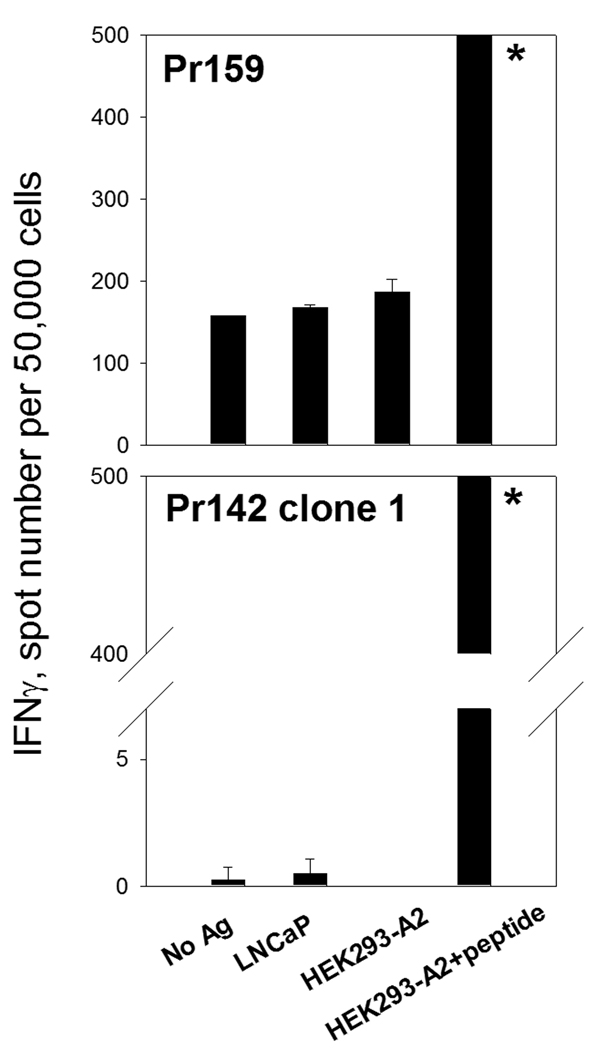
FIGURE 5. Failure of LNCaP cells recognition by peptide-specific CD8 T cell cultures.
The PSA154–165(155L) peptide-specific CD8 T cell cultures were developed as described in Materials and Methods (two rounds of stimulation for Pr159 or multiple stimulations with peptide and cloning for Pr142 clone 1). T cells were co-cultured with irradiated HEK293-A2 cells in the presence or absence of the specific peptide or LNCaP cells for 48 hr. Representative experiments are shown. * Too numerous to count.
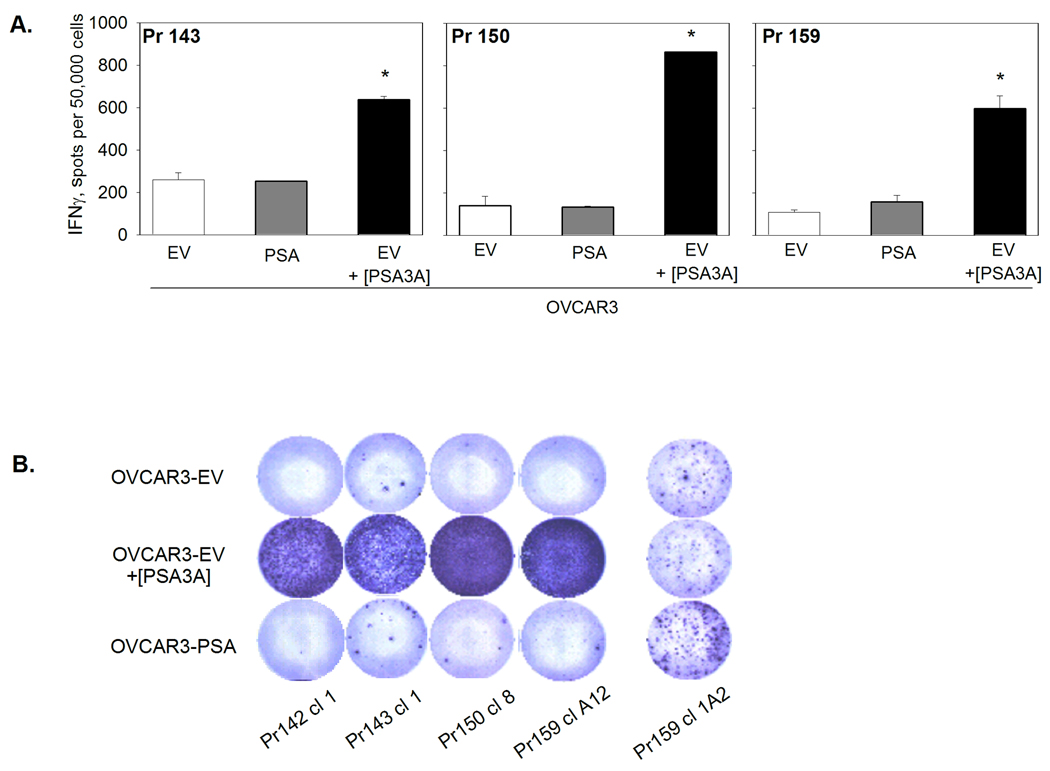
FIGURE 7. Lack of recognition of endogenously-expressed PSA by PSA3A peptide-specific CD8 T cells.
A. PSA3A peptide-specific CD8 T cell lines derived by two cycles of in vitro stimulation with PSA3A peptide were tested for the reactivity with OVCAR3-EV in the presence or absence of specific peptide, or OVCAR3-PSA cells in IFN-γ ELISPOT assay. Results for CD8 T cell lines from three responding patients are shown. Data are mean ±SD of triplicate determinations. * p<0.0001 (2-sided t-test). B. The response of individual clones developed from PSA3A peptide-specific CD8 to PSA3A peptide and PSA-expressing HLA-A2-positive tumor cell line. Peptide-specific CD8 T-cell clones were developed from advanced PSA3A peptide-specific CD8 cultures of all responding patients (Pr142, Pr143, Pr150, Pr159) and tested in IFN-γ ELISPOT assay. Representative clones from each patient are shown.
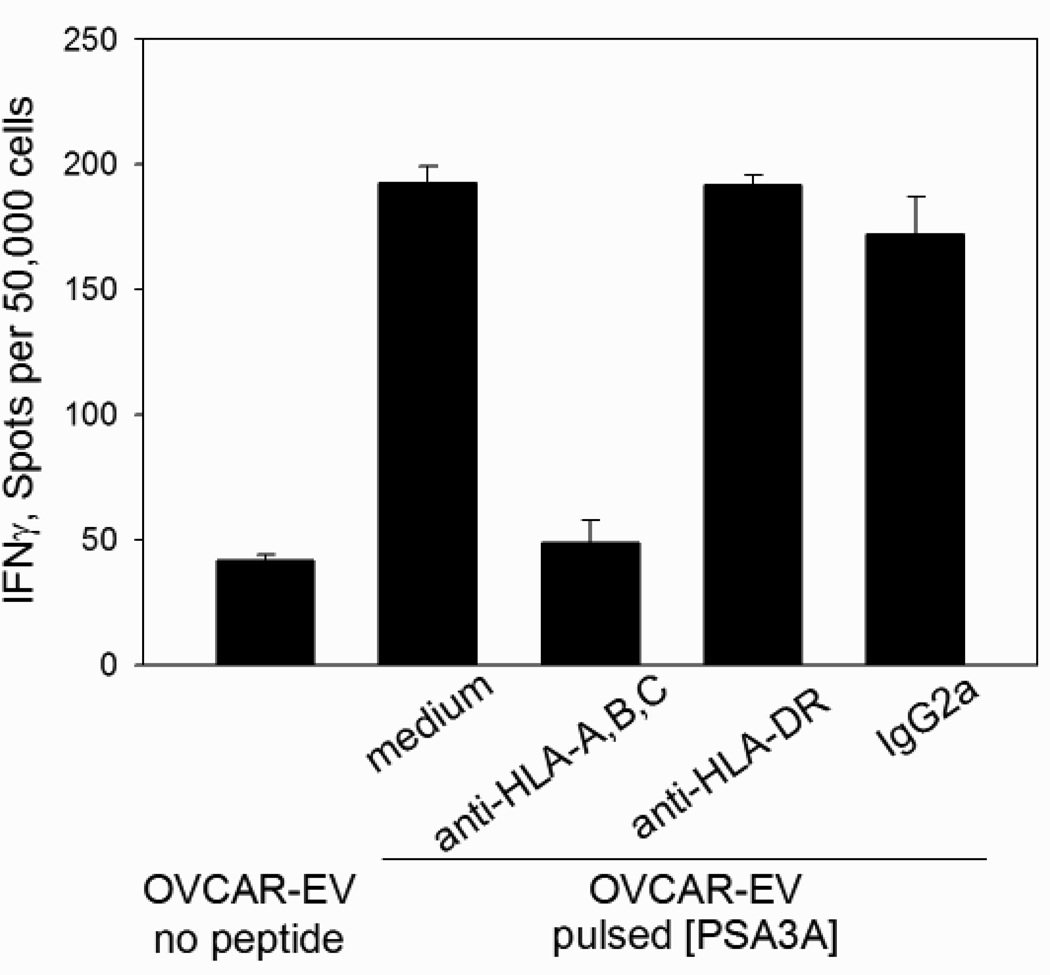
To confirm that CD8 T cells specific to PSA:154–163(155L) peptide are capable of recognition the native PSA sequence, the cross-reactivity with the native PSA:154–163 peptide was tested. All cultures showed strong reactivity with both agonist and native peptides presented by HLAA* 0201-positive target cells (Figure 3). CD8 T cell recognition of PSA:154–163(155L) peptide was MHC class I-restricted, as the response could be inhibited by anti-MHC class I mAb, but not by MHC class II -specific or isotype control mAb (Figure 4).
FIGURE 3. Comparison of the response to PSA3A and native PSA3 peptide.
CD8 T cell cultures from three responding patients were generated as described in Figure 2. A. CD8 T cells were co-cultured with irradiated HEK293-A2 cells in the absence of peptide (gray bars) or presence of PSA3A (black bars) or PSA3 (white bars) peptide (25 µg/ml). B. CD8 T cells from patient Pr159 were co-cultured with HEK293-A2 cells in the presence of increasing concentrations of peptide PSA3A (black circles) or PSA3 (white circles). Frequency of IFN-γ-producing cells was assessed by ELISPOT assay. The data represent mean ±SD of triplicate determinations.
FIGURE 4. MHC restriction of PSA3A peptide-specific CD8 T cell response.
CD8 T cell line from patient Pr150 was tested in IFN-γ ELISPOT assay against HLA-A2 expressing OVCAR3 tumor cells pulsed with PSA3A peptide in the presence of anti-HLA class I, anti-HLA-DR or isotype control antibodies. The data are mean ±SD of triplicate determinations.
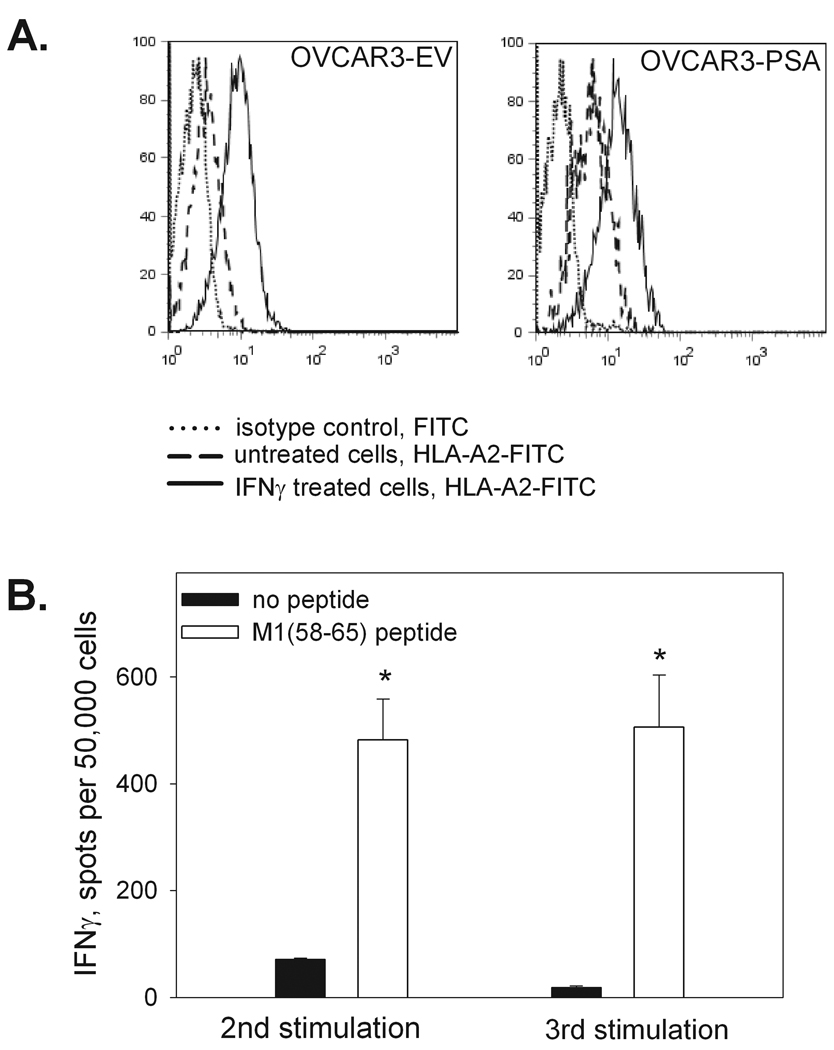
To determine whether the PSA:154–163(155L) peptide-specific CD8 T cell lines could recognize tumor cells expressing endogenous PSA, functional activity of the CD8 T cells was assessed in IFN-γ ELISPOT assays using various PSA-expressing HLA-A2+ target cells. While strong IFN-γ responses were detected when the PSA:154–163(155L) peptide was presented by HLAA* 0201-expressing HEK293 cells, none of the peptide-specific T cell lines could recognize HEK293-A*0201 cells expressing endogenous PSA (HEK293-A2-PSA, Figure 2). Recognition of the HEK293-A2-PSA cell line by Pr150 cultures after two rounds of stimulation was not assessed in the experiment shown in Figure 2, but additional experiments confirmed the lack of the response to HEK293-A2-PSA cells in this patient (data not shown).We also failed to detect any response to PSA-producing prostate carcinoma cell line LNCaP (Figure 5). Although the LNCaP cell line was developed from a HLA-A2 positive patient, HLA-A2 was barely detectable by flow cytometric analysis, and could not be up-regulated by rhIFN-γ treatment (data not shown). Therefore, we used another human tumor cell line, OVCAR3, which is an ovarian cancer cell line produced from a HLA-A*0201+ patient. OVCAR3 expresses high levels of HLA-A2 after treatment with rhIFN-γ (Figure 6A). The PSA level secreted by the OVCAR3-PSA cells after stable transfection with PSA gene-containing plasmid and subsequent cloning was comparable with that of LNCaP cells (OVCAR-PSA cells - 45 ng/106 cells in 24 hr, LNCaP cells – 90 ng/106 cells in 24 hr). To demonstrate that the OVCAR cell line was capable of processing and presenting endogenous antigens, OVCAR3-EV or OVCAR3-PSA cells were infected with influenza A virus. CD8 T cell lines specific to HLA-A2-restricted influenza A virus matrix protein M1 peptide demonstrated strong reactivity with virus-infected OVCAR target cells in the IFN-γ ELISPOT assay (Figure 6B). In contrast, CD8 T cell lines specific to PSA:154–163(155L) peptide demonstrated minimal response to PSA-expressing OVCAR cells and such low level reactivity was observed only in a minority of cultures (Figure 7A and Table 3).
FIGURE 6. Characterization of OVCAR3-PSA target cell line.
A. HLA-A2 expression. OVCAR3-EV and OVCAR3-PSA cell lines were incubated for 48 hr with or without rhIFN-γ (5ng/ml), harvested and stained with anti-HLA-A2-FITC monoclonal antibody or FITC-labeled isotype control antibody. B. Presentation of the endogenously-processed viral peptide. CD8 T cell line was developed from Pr159 PBMC by stimulation with influenza M1matrix protein 58–65 peptide as described in the legend to Figure 2 and tested for the reactivity with influenza A virus-infected OVCAR3-EV (depicted) or OVCAR-PSA (not shown) cells in the IFN-γ ELISPOT assay. Data are mean ±SD of triplicate determinations. * p<0.0001 (2-sided t-test).
Table 3. CTL activity of PSA3A-specific CD8 T cells in Granzyme B ELISPOT assay.
PSA3A peptide-specific CD8 T cell line developed from patient’s Pr159 PBMC was tested for the reactivity with either specific peptide or PSA-expressing tumor cells in IFN-γ or Granzyme B ELISPOT assays. Data represent spots per indicated number of cells. Data are mean ±SD of triplicate determinations. TNTC –"too numerous to count".
| Stimuli | IFN-γ | GRANZYME B | |||
|---|---|---|---|---|---|
| 5×104/well | 2×105/well | 1×105/well | 5×104/well | 2.5×104/well | |
| OVCAR-EV | 59±12 | 37±3 | 22±6 | 13±4 | 5±1 |
| OVCAR-EV PEPT PULSED |
408±52 | TNTC | TNTC | 239±35 | 70±18 |
| OVCAR-PSA | 147±14 | 60±19 | 52±5 | 29±8 | 14±5 |
| medium | 36±5 | 22±7 | 30±3 | 13±2 | 5±1 |
In addition to INF-γ secretion, CTL function was accessed using the granzyme B ELISPOT assay. PSA:154–163(155L) peptide-specific T cell line developed from patient Pr159 PBMC was co-cultured with IFN-γ-treated OVCAR3-PSA or peptide-pulsed OVCAR3-EV cell at different responder/stimulator ratios (4:1, 2:1, 1:1 and 0.5:1). The response to OVCAR3-EV cells in the absence of peptide served as a negative control. The results are presented in Table 3. Similarly to the IFN-γ ELISPOT assay, PSA:154–163(155L)-peptide specific T cell lines demonstrated minimal reactivity with PSA-expressing OVCAR tumor cell line compared to the peptide-pulsed OVCAR-EV cells in the granzyme B ELISPOT assay.
Since we detected a weak response to PSA-expressing OVCAR tumor cells in some cultures, we developed PSA:154–163(155L)-specific CD8 T cell clones by limiting dilution from CD8 T cell bulk cultures. Individual clones were then tested for the reactivity with PSA:154–163(155L) peptide or PSA-expressing OVCAR cells in IFN-γ ELISPOT assay. None of the clones that showed strong response to the peptide-pulsed target cells could recognize target cells expressing endogenous PSA. Presented on Figure 7B are images of representative wells from selected CD8 T cell clones developed from patients Pr142, Pr143, Pr150, Pr159. Interestingly, on a rare occasion we observed clones that reacted with the PSA-expressing tumor cells, but were not reactive with the PSA:15–163(155L) peptide (Figure 7B, clone 159cl1A2 shown).
Discussion
We report the results of a clinical trial of a peptide vaccine based on peptide PSA:154–163(155L) (VLSNDVCAQV) (PSA3A) for the treatment of recurrent prostate cancer. PSA:154–163(155L) was identified as an agonist human T-lymphocyte epitope of PSA and has higher affinity of binding to HLA-A2 molecules compared to the native peptide.26 The primary objective of the protocol was to determine if vaccination with PSA:154–163(155L) and adjuvant can increase the T lymphocyte immune response to PSA:154–163(155L) and processed whole PSA in HLA-A2+ patients with prostate cancer. The end point for the immunologic analyses for this study was based on IFN-γ ELISPOT assays performed on cryopreserved PBMC at baseline and after completion of vaccination. We also tested the reactivity with PSA:154–163(155L) peptide on fresh unfractioned PBMC at different time points during the vaccination course. Five patients each received total of seven vaccinations with the peptide. None of the patients showed immunoreactivity against the peptide in the primary PBMC assays either before or after vaccination.
To amplify and detect possible immune response to the vaccinations in our study, we developed PSA:154–163(155L)-specific CD8 T cell lines by repeated stimulation with PSA:154–163(155L) peptide, and tested the cell lines for reactivity with specific agonist peptide, native PSA:154–163 peptide and tumor cell lines expressing endogenous PSA. Analysis of the CD8 T cell response after one or two cycles of in vitro stimulation indicated that in four of five patients the vaccination produced peptide-reactive CD8 T lymphocytes. We did not detect any response in pre-vaccination CD8 T cell cultures, thus, positive responses in post-vaccination cultures are the result of exposure to the vaccine. These results can be considered as evidence of a successful vaccination in these patients; however, such low frequencies of antigen-specific cells could not be detected by direct ELISPOT assay using unfractioned PBMC. Since the response can only be detected after CD8 T cell enrichment and 1–2 rounds of amplification in vitro, the vaccine was not very immunogenic, at least in the combination with Montanide ISA-51 adjuvant. PSA3 and PSA:154–163(155L) peptides were initially identified using PBMC from non-vaccinated HLAA2 positive donors 24, but the reactivity was detected after 3 cycles of in vitro stimulation with PSA:154–163(155L) peptide 26, and 6–7 cycles were required in order to achieve detectable responses to PSA:154–163 peptide.24 Similar results of a clinical trial with autologous dendritic cells loaded with peptides PSA-1 [141–150]; PSA-2 [146–156]; PSA-3 [154–163] in patients with hormone refractory prostate carcinoma have been reported recently by Hildenbrand et al 33. Direct ex vivo IFN-γ ELISPOT assay and the MHC class I multimer analysis detected PSA3-peptide specific CTL at very low frequencies (3–5 spots/106 CD8 T cells) in two of thirteen patients after completion of four vaccinations.
One of the approaches used to attempt to increase the effectiveness of peptide vaccines has been to alter the amino acid sequence of immunogenic peptides to increase HLA binding affinity. Several modified peptides derived from melanoma were shown to be more efficient than the native peptide in inducing specific immune response (Melan-A26–35 analogues 34, gp100209-2M 35). A modified peptide gp100209-2M exhibited increased binding to HLA-A2 molecules and had an increased ability to generate melanoma reactive CTLs after stimulation of the PBMC of HLAA2+ patients.36 Clinical studies of gp100209-2M peptide have shown that vaccination with the peptide gp100209-2M results in significant CD8+ T-cell immune response and can mediate tumor regression in patients with metastatic melanoma at a rate higher than native gp100 peptide. 35,37 PSA:154–163(155L) agonist peptide was chosen for use as vaccine in the trial because this modified peptide has been shown to be superior to native peptide PSA:154–163 in terms of binding affinity to MHC molecules and was more efficient in T cells activation in vitro and in vivo than the native peptide 26. We assessed the ability of the PSA:154–163(155L)-specific CTLs to recognize native peptide PSA:154–163 and confirmed previously reported data that T cells induced after immunization with a modified peptide are able to recognize the native peptide.26 However, we did not succeed in attempts to demonstrate specific cytotoxic activity of the PSA:154–163(155L)-specific cell lines against PSA-expressing HLA-A2 positive tumor cells. A low response to the PSA-producing cells was detected only in CD8 T cell cultures developed from patient Pr159 PBMC. However, we could not confirm this at the clonal level, since none of the T cell clones could recognize both the specific peptide and PSA-expressing tumor cells in the same experiment. These data suggest that low levels of PSA reactivity that we could detect in some of the cultures were probably due to the non-specific reaction to the allogeneic target cells and were not related to the specific anti-PSA immune response. These findings provide evidence that the native peptide, PSA:154–163, is not effectively processed and presented by PSA-expressing tumor cells. Choice of the method of detecting the antigen-specific T-cells is essential for correct detection of tumor-recognizing cells. Granzyme B is one of the most detectable mediators of target cell death in CTL and it’s release in response to the specific target may be used to evaluate cell-mediated cytotoxicity by specific antitumor CTL generated by vaccination.38 Our results of the GrB ELISPOT assay were in concordance with IFN-γ assay and further confirmed absence of PSA-expressing target cells recognition by PSA:154–163(155L) peptide specific CD8 T cell lines. The OVCAR cell line used as a target in our experiments was rigorously tested and shown to express high levels of HLA-A2 after treatment with IFN-γ and secreted significant amount of PSA comparable to LNCaP cells. Using a model antigen (influenza virus) we also proved this line to be capable of efficient processing and presentation of the endogenously-expressed antigens. However, PSA:154–163(155L) peptide specific CD8 T cell lines uniformly did not significantly respond to OVCAR-PSA.
Correale et al 24 described the recognition of PSA:154–163 peptide using in vitro stimulation of PBMC. The authors showed evidence of recognition of endogenous PSA using a vaccinia virus-PSA construct and cold-target inhibition studies. PSA-secreting HLA-A2 positive target cell line prostate carcinoma LNCaP cells were used as target cells in these experiments. The PSA:154–163-specific effector cells lysed LNCaP cells in cytotoxic assays. In later studies the same group showed in cold target inhibition experiments that a longer oligopeptide containing PSA-1 and PSA:154–163 peptide sequences could generate T cells that recognized HLA-A2 antigen-presenting cells endogenously synthesizing PSA.39 This provided evidence that the PSA3 epitope is naturally processed and presented. In the paper describing agonist PSA:154–163(155L) peptide, Terasawa et al. showed specific lysis of LNCaP cells by the peptide-specific cultures.26 However, we could not detect any recognition of the LNCaP cells by the patients’ cultures in a sensitive IFN-γ ELISPOT assay, though they strongly responded to the stimulation with the peptide presented by other peptide-pulsed tumor cell lines. The LNCaP cell line is commonly used as a model cell line in prostate cancer studies. However, several studies indicated that HLA class I is underexpressed on LNCaP, rendering the cells poor at presenting antigens in the context of class I. In addition, the LNCaP cell line is unresponsive to IFN-γ treatment due to the lack of JAK1 gene expression.40,41
Secondary outcomes of the clinical study were determination of toxicity and effect on serum PSA. The vaccine was in general well tolerated, with only grade 1 or grade 2 adverse events reported. Serum PSA levels rose slightly in 2 patients and were unchanged in 3 patients. All patients had rising PSA at the time of study enrollment. Hence, it is not possible to determine if the vaccinations were responsible for the rise in PSA seen in these two patients or if the increase was due to the expected natural history of their disease. In any event no significant decreases in serum PSA were observed in any patient.
In summary, we show that PSA:154–163(155L) peptide vaccination with Montanide ISA-51 can induce weak immune responses to the peptide vaccine that were observed only after in vitro stimulation with the peptide. Despite these responses to the agonist and native peptide sequences, we were unable to demonstrate significant responses to any of several tumor cell lines expressing native PSA, even though the tumor cells were shown to be capable of processing and presenting endogenous influenza peptide epitopes. These data show that PSA:154–163 is a cryptic epitope and is poorly presented by PSA-expressing tumor cells.
Acknowledgments
Supported by NCI Cancer Therapy Evaluation Program protocol #6991 and the University of Maryland General Clinical Research Center Grant M01 RR 16500, General Clinical Research Centers Program, National Center for Research Resources (NCRR), NIH.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
REFERENCES
- 1.Holmberg L, Bill-Axelson A, Helgesen F, et al. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med. 2002;347:781–789. doi: 10.1056/NEJMoa012794. [DOI] [PubMed] [Google Scholar]
- 2.Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 3.Walsh PC. Surgery and the reduction of mortality from prostate cancer. N Engl J Med. 2002;347:839–840. doi: 10.1056/NEJMe020089. [DOI] [PubMed] [Google Scholar]
- 4.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 5.Oesterling JE, Chan DW, Epstein JI, et al. Prostate specific antigen in the preoperative ond postoperative evaluation of localized prostatic cancer treated with radical prostatectomy. J Urology. 1988;139:766–772. doi: 10.1016/s0022-5347(17)42630-9. [DOI] [PubMed] [Google Scholar]
- 6.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18:175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 9.Boon T, Coulie PG, Van den EB. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 10.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- 11.Bettinotti MP, Panelli MC, Ruppe E, et al. Clinical and immunological evaluation of patients with metastatic melanoma undergoing immunization with the HLA-Cw*0702-associated epitope MAGE-A12:170–178. Int J Cancer. 2003;105:210–216. doi: 10.1002/ijc.11045. [DOI] [PubMed] [Google Scholar]
- 12.Weber JS, Hua FL, Spears L, et al. A phase I trial of an HLA-A1 restricted MAGE-3 epitope peptide with incomplete Freund's adjuvant in patients with resected high-risk melanoma. J Immunother. 1999;22:431–440. doi: 10.1097/00002371-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Cebon J, Jager E, Shackleton MJ, et al. Two phase I studies of low dose recombinant human IL-12 with Melan-A and influenza peptides in subjects with advanced malignant melanoma. Cancer Immun. 2003;3:7. [PubMed] [Google Scholar]
- 14.Wang F, Bade E, Kuniyoshi C, et al. Phase I trial of a MART-1 peptide vaccine with incomplete Freund's adjuvant for resected high-risk melanoma. Clin Cancer Res. 1999;5:2756–2765. [PubMed] [Google Scholar]
- 15.Scheibenbogen C, Schadendorf D, Bechrakis NE, et al. Effects of granulocyte-macrophage colony-stimulating factor and foreign helper protein as immunologic adjuvants on the T-cell response to vaccination with tyrosinase peptides. Int J Cancer. 2003;104:188–194. doi: 10.1002/ijc.10961. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JJ, Janetzki S, Schaed S, et al. Evaluation of CD8(+) T-cell frequencies by the Elispot assay in healthy individuals and in patients with metastatic melanoma immunized with tyrosinase peptide. Int J Cancer. 2000;87:391–398. doi: 10.1002/1097-0215(20000801)87:3<391::aid-ijc13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Scheibenbogen C, Schmittel A, Keilholz U, et al. Phase 2 trial of vaccination with tyrosinase peptides and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma. J Immunother. 2000;23:275–281. doi: 10.1097/00002371-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Slingluff CL, Jr, Yamshchikov G, Neese P, et al. Phase I trial of a melanoma vaccine with gp100(280–288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–3024. [PubMed] [Google Scholar]
- 19.Lee P, Wang F, Kuniyoshi J, et al. Effects of interleukin-12 on the immune response to a multipeptide vaccine for resected metastatic melanoma. J Clin Oncol. 2001;19:3836–3847. doi: 10.1200/JCO.2001.19.18.3836. [DOI] [PubMed] [Google Scholar]
- 20.Weber J, Sondak VK, Scotland R, et al. Granulocyte-macrophage-colony-stimulating factor added to a multipeptide vaccine for resected Stage II melanoma. Cancer. 2003;97:186–200. doi: 10.1002/cncr.11045. [DOI] [PubMed] [Google Scholar]
- 21.Schaed SG, Klimek VM, Panageas KS, et al. T-cell responses against tyrosinase 368–376(370D) peptide in HLA*A0201+ melanoma patients: randomized trial comparing incomplete Freund's adjuvant, granulocyte macrophage colony-stimulating factor, and QS-21 as immunological adjuvants. Clin Cancer Res. 2002;8:967–972. [PubMed] [Google Scholar]
- 22.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue BH, Zhang Y, Sosman JA, et al. Induction of human cytotoxic T lymphocytes specific for prostate- specific antigen. Prostate. 1997;30:73–78. doi: 10.1002/(sici)1097-0045(19970201)30:2<73::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Correale P, Walmsley K, Nieroda C, et al. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. Journal of the National Cancer Institute. 1997;89:293–300. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 25.Hodge JW, Schlom J, Donohue SJ, et al. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int J Cancer. 1995;63:231–237. doi: 10.1002/ijc.2910630215. [DOI] [PubMed] [Google Scholar]
- 26.Terasawa H, Tsang KY, Gulley J, et al. Identification and characterization of a human agonist cytotoxic T- lymphocyte epitope of human prostate-specific antigen. Clin Cancer Res. 2002;8:41–53. [PubMed] [Google Scholar]
- 27.Alexander RB, Brady F, Leffell MS, et al. Specific T cell recognition of peptides derived from prostate specific antigen in patients with prostatic cancer. Urology. 1998;51:150–157. doi: 10.1016/s0090-4295(97)00480-9. [DOI] [PubMed] [Google Scholar]
- 28.Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 29.Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 30.Arlen PM, Gulley JL, Tsang KY, et al. Strategies for the development of PSA-based vaccines for the treatment of advanced prostate cancer. Expert Rev Vaccines. 2003;2:483–493. doi: 10.1586/14760584.2.4.483. [DOI] [PubMed] [Google Scholar]
- 31.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 32.Malyguine A, Strobl SL, Shafer-Weaver KA, et al. A modified human ELISPOT assay to detect specific responses to primary tumor cell targets. J Transl Med. 2004;2:9. doi: 10.1186/1479-5876-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hildenbrand B, Sauer B, Kalis O, et al. Immunotherapy of patients with hormone-refractory prostate carcinoma pre-treated with interferon-gamma and vaccinated with autologous PSA-peptide loaded dendritic cells--a pilot study. Prostate. 2007;67:500–508. doi: 10.1002/pros.20539. [DOI] [PubMed] [Google Scholar]
- 34.Valmori D, Fonteneau JF, Lizana CM, et al. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- 35.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [see comments] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkhurst MR, Salgaller ML, Southwood S, et al. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 37.Walker EB, Haley D, Miller W, et al. gp100(209-2M) peptide immunization of human lymphocyte antigen-A2+ stage I-III melanoma patients induces significant increase in antigen-specific effector and long-term memory CD8+ T cells. Clin Cancer Res. 2004;10:668–680. doi: 10.1158/1078-0432.ccr-0095-03. [DOI] [PubMed] [Google Scholar]
- 38.Shafer-Weaver K, Sayers T, Strobl S, et al. The Granzyme B ELISPOT assay: an alternative to the 51Cr-release assay for monitoring cell-mediated cytotoxicity. J Transl Med. 2003;1:14. doi: 10.1186/1479-5876-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Correale P, Walmsley K, Zaremba S, et al. Generation of human cytolytic T lymphocyte lines directed against prostate-specific antigen (PSA) employing a PSA oligoepitope peptide. J Immunol. 1998;161:3186–3194. [PubMed] [Google Scholar]
- 40.Sanda MG, Restifo NP, Walsh JC, et al. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–285. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn GP, Sheehan KC, Old LJ, et al. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 2005;65:3447–3453. doi: 10.1158/0008-5472.CAN-04-4316. [DOI] [PubMed] [Google Scholar]