Figure 6.
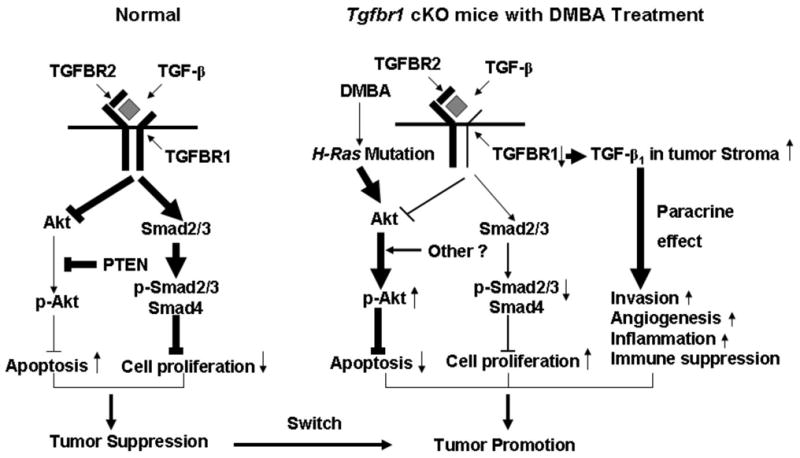
A schematic presentation of the proposed TGF-β signaling alteration that promotes HNSCC in mice. In normal cells, TGF-β inhibits cell proliferation through the Smad-dependent pathway. It also induces apoptosis by repressing the PI3K/Akt pathway results in tumor suppression. Decreased Tgfbr1 expression in Tgfbr1 cKO mice leads to increased cell proliferation and cell survival through PTEN independent activation of the PI3K/Akt pathway. DMBA treatment, which causes H-ras mutation as well as other mechanisms, may also play an important role in Akt activation. Decreased TGFBR1 can also increase TGF-β1 in tumor stroma by as yet unidentified mechanisms, which leads to increased invasion, angiogenesis, and inflammation, as well as immune suppression through the paracrine effect of TGF-β. In summary, inactivation of TGF-β signaling, in the context of ras mutations and aberrant activation of the PI3K/Akt pathway and accompanied by increased paracrine effect of TGF-β, switches TGF-β signaling from tumor suppression in normal cells to tumor promotion in head and neck carcinogenesis of Tgfbr1 cKO mice.
