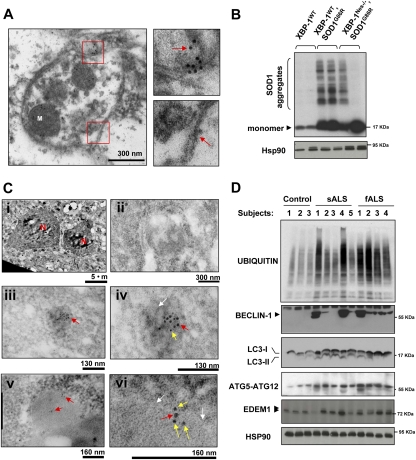
Figure 7.
Decreased mutant SOD1 aggregation in XBP-1-deficient mice and enhanced autophagy in sALS and fALS patients. (A) Electron microscopy sections of SOD1G86R/XBP-1Nes−/− mice were analyzed by immunogold staining of SOD1. Autophagosomes were identified by the presence of double-membrane vesicles containing cytosol and organelles such as mitochondria (M). (Bottom panel) A higher magnification of the areas marked with red squares is shown to depict the double membrane of the vesicle and SOD1 immunogold staining (see arrows). Images are representative of three independent experiments. (B) SOD1 aggregation was determined by Western blot in XBP-1Nes−/− and control SOD1G86R presymptomatic female mice of the same litter (90 d of age). Two animals per group are presented. As loading control, Hsp90 levels were monitored. (C) Colocalization of SOD1 and LC3 was determined by double-immunogold staining. Gold spheres were 5 nm (SOD1) and 10 nm (LC3). (Panel i) Low-magnification picture to identify motoneurons by their size, morphology, and location in the spinal cord. (N) Nucleus. (Panel ii) Negative control for staining. (Panels iii,iv) Two magnifications showing the colocalization of LC3 and SOD1 inside a dense structure (white arrow). (Panels v,vi) Two magnifications showing the colocalization of LC3 and SOD1 in an autophagosomal structure. White arrows indicate double membranes. Arrows in panels iv and vi are SOD1 (yellow), 5-nm gold colloid; and LC3 (red), 10-nm gold colloid. Representative pictures of the analysis of three animals per group in two independent experiments are presented. (D) Increased levels of autophagy in post-mortem samples of sALS and fALS cases. The levels of BECLIN-1, ATG5–ATG12 complex, LC3, EDEM1, and polyubiquitinated proteins were investigated in total protein extracts derived from spinal cord post-mortem samples from sALS and fALS patients and three healthy control subjects. HSP90 levels were analyzed as loading control.