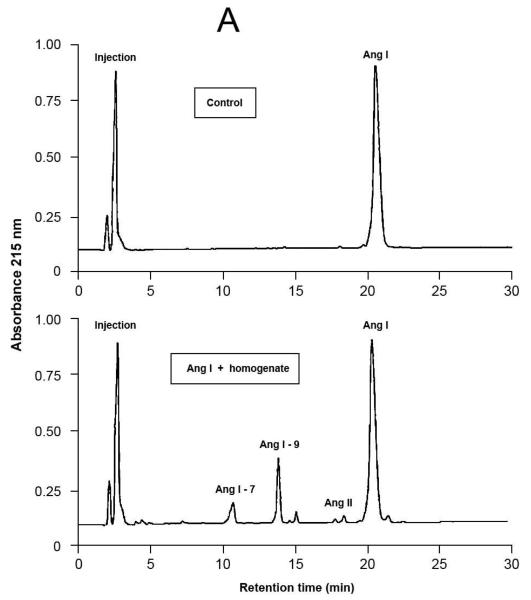
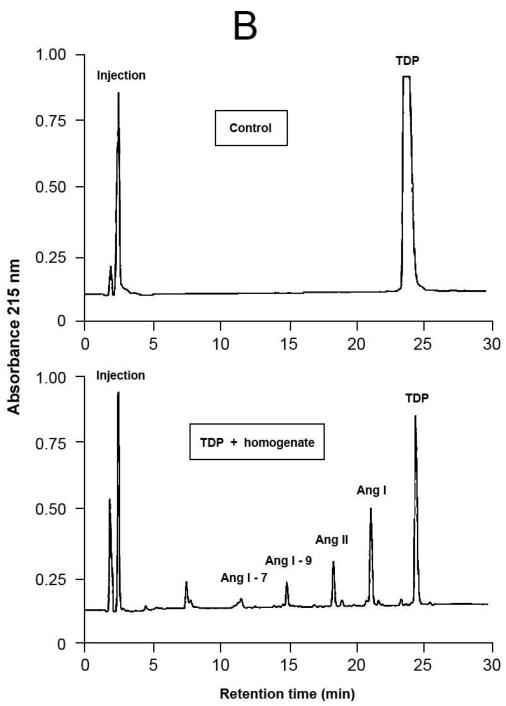
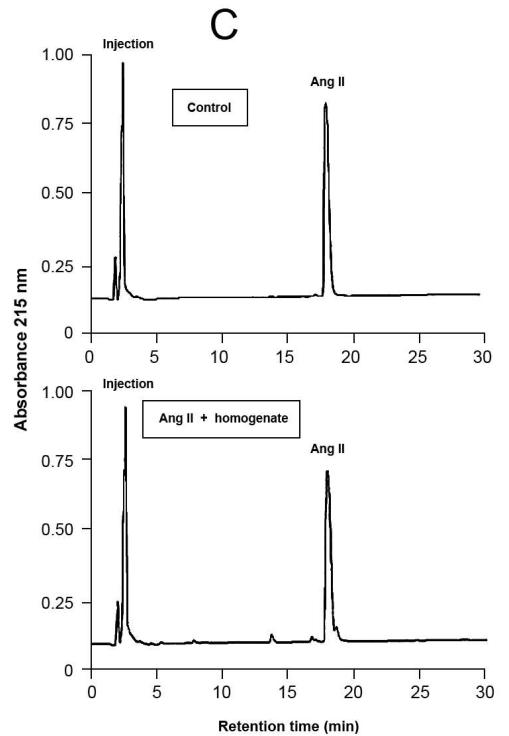
Fig.4.
Representative results of proteolytic cleavage of angiotensin (Ang) I [A], renin substrate tetradecapeptide (TDP) [B] and Ang II [C] catalyzed by rat gingival tissue homogenate. Homogenate sample was incubated with either Ang I (30 nmol), TDP (25 nmol) or Ang II (30 nmol), in a final volume of 150 μL, in Tris buffered saline (0.03 M Tris-HCl containing 0.15 M NaCl, pH = 8.1) at 37°C for 20 min. Enzymatic reaction was stopped with the addition of 40 μL of 5% trifluoroacetic acid in 4% glycerol. Upper panels show the substrates without the incubation with the homogenate (control), while the lower panels show the hydrolysis products of the substrates by enzymatic activities present in the homogenate. Chromatograms were generated by reversed-phase HPLC analysis on a C-18 column (4 × 250 mm) developed at a flow rate of 1.0 mL/min with a linear gradient of acetonitrile (12–32%, 30 min) in 0.1% TFA. Peptides were detected by absorbance at 215 nm and quantified by comparison with known concentrations of cognate synthetic substrates.