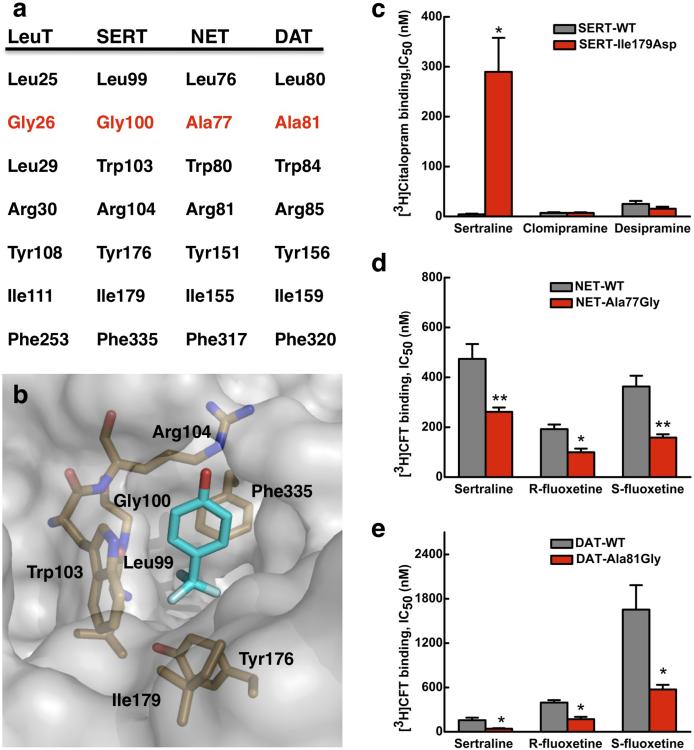
Figure 3. Halogen-binding pocket in SERT and its critical importance in the protein's specificity for antidepressant.
a. Sequence alignment at the HBP between NSS proteins. The only difference in primary sequence between SERT and NET or DAT is at Gly100 in SERT, which is an alanine in the latter two proteins. b. Docking of R-fluoxetine to a SERT homology model 18. Only the trifluoromethylphenoxy group of the drug is shown for clarity. c – e. Changes in affinity (IC50) for antidepressants of human SERT, NET and DAT when a residue at their HBP is mutated, relative to the appropriate wild-type transporter, as measured in binding assays with HEK293 cells. c. Binding of [3H]citalopram to the SERT-Ile179Asp mutant in the presence of sertraline, chlomipramine and desipramine. d. Binding of [3H]CFT to the NET-Ala77Gly mutant and to the DAT-Ala81Gly mutant (e) both in the presence of sertraline, R-fluoxetine and S-fluoxetine. Values are the Mean ± S.E.M. of three to five experiments. The significance of measurements is indicated by * (P < 0.05) and ** (P < 0.005) compared with wild-type (Student's t-test).