Abstract
Purpose
The aim of this study is to determine whether inclusion of caspase inhibitor can improve the efficacy of cryopreservation of ovarian tissue.
Methods
Mice were randomly assigned to the Group A (fresh control group) Group B (inclusion of caspase inhibitor) and Group C (non-inclusion of caspase inhibitor). Ovarian tissue in Group B and Group C was vitrified-thawed. TUNEL assay and Bax protein detection were measured after cryopreservation. The mice in all groups received autotransplantation. The number of days before the resumption of estrous cycles was measured daily from the 5th day after surgery, and the percentage of cells expressing PCNA in grafts was measured one month following transplantation.
Results
The incidence of TUNEL positive follicles in Group B was significantly higher than that in Group C. Similarly, the percentage of follicles expressing Bax protein in Group B was significantly higher than that in Group C. The number of days before the resumption of estrous cycles in Group B was significantly less than that in Group C. In addition, the percentage of follicular and stromal cells expressing PCNA of grafts in Group B was significantly higher than that in Group C.
Conclusions
The global caspase inhibitor Z-VAD-FMK decreases the incidence of apoptosis of ovarian tissue induced by cryopreservation, and inclusion of caspase inhibitor improves the efficacy of cryopreservation of ovarian tissue.
Keywords: Caspase inhibitor, Z-VAD-FMK, Cryopreservation, Ovarian tissue
Introduction
Advances in cancer therapy have enabled long-term remission and even cure of many cancers. Most cancer therapeutic agents are cytotoxic. Because germ cells are particularly susceptible to cytotoxic treatments (especially alkylating agents and radiation), ovarian failure and infertility are common complications that can impact on the quality of life for young survivors of cancer [1, 2]. An emerging technology, involving transplantation of ovarian cortical slices banked at low temperature offers the possibility of restoring fertility as well as endocrine function in women and children after sterilizing treatment [3]. However, it is still experimental in humans and raises both technical and ethical issues to be resolved.
It was reported that at least 70% of primordial follicles in the graft tissue are lost as a result of cryopreservation damage and ischemia during graft revascularization [4]. The loss of viability of cells and follicles following cryopreservation was at least partly attributed to apoptosis [5]. It was suggested that increased apoptosis in follicles after cryopreservation was probably caused by physical alteration due to low temperature, high salt concentrations, and an impaired antioxidant metabolism [6]. The caspase family of cysteine proteases serves as the central executioners of apoptosis. Once activated, caspases induce cells to undergo apoptosis by cleaving and altering the function of diverse intracellular proteins. The inhibition of caspase pharmacologically can block the completion of apoptosis [7].
In this current study, it was hypothesized that apoptosis may be induced by cryopreservation and the inclusion of a global caspase inhibitor (Z-VAD-FMK) in the cryopreservation media may act to prevent some of this apoptosis and hence improve tissue survival.
The aim of the present study, therefore, was to determine whether inclusion of a global caspase inhibitor (Z-VAD-FMK) can relieve apoptosis caused by cryopreservation and hence improve the efficacy of cryopreservation of ovarian tissue.
Materials and methods
Chemicals were purchased from Sigma-Aldrich Company (St. Louis, MO, USA) unless otherwise indicated.
Animals and ovarian tissue
4-week-old female mice (ICR, n = 60) were used in this study. The mice used were all healthy and were sourced from Shanghai Animal Center. The mice were housed under temperature-controlled conditions (22 ± 2°C). Food and water were available at all times under a photoperiod of 12 h of light and 12 h of dark. Ovaries were cut into 1 mm × 1 mm × 1 mm slices free of fat and mesentery. Approval for this study was obtained from the Animal Reserch Ethical Committee of the Shanghai Jiao Tong University.
Vitrification and thawing
Cryopreservation solution was prepared in modified phosphate-buffered saline (DPBS) medium (Sigma) supplemented with 15% heated-inactivated fetal calf serum (Sijiqing Co., Hangzhou, China).
The mice (n = 60) were randomly assigned to fresh control group (Group A) and treatment groups. The treatment groups divided into two groups: inclusion of caspase inhibitor (Group B) and non-inclusion of caspase inhibitor (Group C). Group B: equilibration solution was prepared with 10% (v/v) ethylene glycol (EG; Sigma) +10% (v/v) dimethyl sulfoxide (DMSO; Sigma) +60 uM Z-VAD-FMK in DPBS; vitrification solution was prepared with 20%(v/v)EG +20% (v/v) DMSO +60 uM Z-VAD-FMK +0.4 M sucrose (Sigma) in DPBS. Group C: equilibration solution and vitrification solution used for Group C were identical to those for Group B, but without Z-VAD-FMK.
Equilibration and vitrification Ovarian tissue was equilibrated in equilibration solution at room temperature for 20 min and then was placed in vitrification solution at room temperature for 10 min. Finally, the ovarian tissue was placed in 0.25-ml plastic straws (L,Aigle, France) with a minimal volume of vitrification medium and plunged into liquid nitrogen for storage.
Thawing procedure The ovarian tissue was cryopreserved for 30 days. Straws were warmed at room temperature for 20 s and placed in a 25°C water bath for 20 s. The contents of each straw were expelled into thawing media. The thawing media of Group C contained 1 mL of descending concentrations of sucrose (1, 0.5, 0.25 M); the thawing media of Group B was identical to that of Group C , but with inclusion of 60 uM Z-VAD-FMK. The contents were placed in thawing media at room temperature for 5 min at each dilution. The warm strips were rinsed for three times in the base medium and put into a 37°C, 5% CO2 humidified incubator for 30 min for following procedures.
TUNEL assay
All strips were rinsed for three times in the base medium and placed into five organ culture dishes (falcon Cat. No. 3037) containing 1 ml of culture medium. The culture medium was TCM 199 (Gibco BRL) supplemented with 0.06 g/L benzyl penicillin, 0.05 g/L streptomycin, and 0.25 g/L fungi zone (Sigma UK Ltd.) plus 10% FCS. The dishes were incubated at 37°C, 5% CO2 humidified incubator for 6 h for TUNEL assay. Strips (n = 5 for each group) were fixed in 4% phosphate-buffered formaldehyde at 4°C overnight, embedded in paraffin by the standard method, cut into 5-um sections, and mounted on poly-Lysine-coated slides. Tissue sections were deparaffinized by heating at 60°C for 5 min and washing twice in xylene for a total of 10 min. The sections were then rehydrated through a graded series of alcohols and double distilled water. In situ TUNEL analyses were performed according to the instructions of a commercial assay kit (Boshide Co., Wuhan, China). Sections were digested by 20 ug/ml proteinase K in 10 mM Tris-HCL for 30 min at room temperature. The slides were incubated in 50 ul of TUNEL reaction mixture for 1 h at 37°C in a humidified dark chamber. Then the slides were rinsed in PBS three times. Then slides were incubated with converter antifluorescein antibody conjugated with peroxides and diluted to 1:1 with PBS containing 0.05% bovine serum albumin (BSA) for 30 min in a humidified chamber. After washing with PBS two times, immunoreactions were detected by incubating with substrate solution consisting of 0.2 mg/ml 3,3-diaminobenzidine-4HCL and 0.005% H2O2 in 0.05 M Tris-HCL (pH 7.5). The slides were rinsed with water and counterstained with haematoxylin. After washing with running water, the slides were dehydrated, cleared and covered. Negative control sections were incubated with TUNEL reaction mixture without enzyme (TdT). Follicles with positive TUNEL staining of the oocyte and/or ≥50% granulosa cells were considered apoptotic.
Bax protein detection
Tissue samples (n = 5 for each group) were fixed in 10% neutral buffered formalin, routinely processed and embedded in paraffin blocks. Sections mounted on glass slides were deparaffinized and rehydrated through graded alcohols and endogenous peroxidase activity blocked with 3% H2O2 in 70% methanol. The sections were washed as in step 3 for 10 min in phosphate-buffered-saline (PBS, pH 7.3), and non-specific protein-blinding sites were blocked with 3% normal goat serum to reduce background staining. The sections were processed for immunolocalisation of Bax using a commercially available polyclonal antibody against Bax (1:00 dilution, Boshide Co., Wuhan, China) as the primary antibodies. The following morning, the samples were rinsed and incubated with rabbit antimouse biotinylated immunoglubulin (Boshide Co., Wuhan, China) at a dilution of 1:200 for 30 min. And then the samples were processed with 0.05% (w/v) 3,3-diaminobenzidine and 0.01% (v/v) hydrogen peroxide in PBS (10 mM, pH 7.4). These sections were counter-stained with hematoxylin-eosin and mounted with entellan. Screen shots were taken with Camedia digital camera at Olympus BX51 microscope (Olympus, Hamburg, Germany). Expression Bax protein was identified as diffuse golden yellow cytoplasmic staining (sites of the mitochondria) in oocytes or granulosa.
Autotransplantation
Mice (n = 36) were ovariectomized. The mice in control group received fresh ovarian autotransplants, while the mice in experimental groups received frozen-thawed ovarian autotransplants. The kidney was exteriorized through a dorsal-horizontal incision. A small hole was torn in the kidney capsule using fine watchmaker,s forceps under aseptic conditions. Ovarian tissues were inserted under the kidney capsule through the small hole. Both sides of kidneys of each mouse received grafts, and four grafts were autotransplanted to each mouse. Finally, the body wall incisions and skin were closed. The transplantation process was performed at room temperature. The duration of the grafting process was ~30 min in each experimental trial. The transplantation protocol was approved by Animal Reserch Ethical Committee of the Shanghai Jiao Tong University.
PCNA protein detection
At 30 days after surgery, grafts were removed. Tissue sections (5 um) were dewaxed and rehydrated through an ascending series of alcohols before undergoing heat and citrate buffer (10 mM; pH6.0) antigen retrieval. To detect PCNA expression, anti-PCNA mouse monoclonal antibody (Boshide Co., Wuhan, China) was diluted 1:2000 in PBS and incubated on sections overnight at 4°C. Negative controls were also included where the primary antibody was substituted for mouse immunoglobulin (Ig) G at the same concentration. The following morning, the samples were rinsed and incubated with rabbit antimouse biotinylated immunoglobulin at a dilution of 1:200 for 30 min. And then the samples were processed with 0.05% (w/v) 3,3-diaminobenzidine and 0.01% (v/v) hydrogen peroxide in PBS (10 mM, pH 7.4). These sections were counter-stained with hematoxylin-eosin and mounted with entellan. Screen shots were taken with Camedia digital camera at Olympus BX51 microscope (Olympus, Hamburg, Germany).
Vaginal smear examination
Vaginal smears were taken daily, starting on day 5 postsurgery, from all mice in groups. Using sterile pipettes and sterile normal saline, the vagina of each mouse was flushed gently and the cells were smeared onto a slide. The stage of the estrous cycle was determined from the cell types observed in the smear [8], the appearance of cornified epithelial cells (>15%) was considered as initiation of estrous cycle, the survival and functional recovery of grafts were defined as at least two consecutive 4-day estrous cycles showing.
Statistical analysis
Statistical comparisons were carried out using analysis of variance (ANOVA) and x2 test. Values were considered significant when P < 0.05. SAS version 8.1 software (SAS Institute, Cary, NC, USA) was used for all statistical analysis.
Results
TUNEL assay
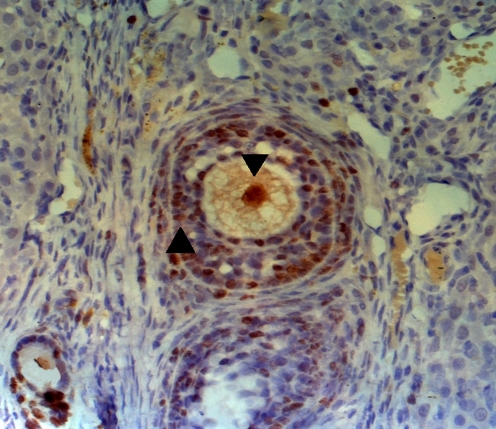
The incidence of TUNEL positive follicles in Group A (fresh control group) was significantly lower than those in Group B (inclusion of caspase inhibitor) and Group C (non-inclusion of caspase inhibitor) (16.71 ± 2.12% versus 23.25 ± 2.51% and 29.78 ± 3.92%, P < 0.05). The incidence of TUNEL positive follicles in Group B (inclusion of caspase inhibitor) was significantly higher than that in Group C (non-inclusion of caspase inhibitor) (23.25 ± 2.51% versus 29.78 ± 3.92%, P < 0.05) (Fig. 1).
Fig. 1.
The TUNEL positive oocyte (▼), the TUNEL positive granulosa cell (▲)
Bax protein detection
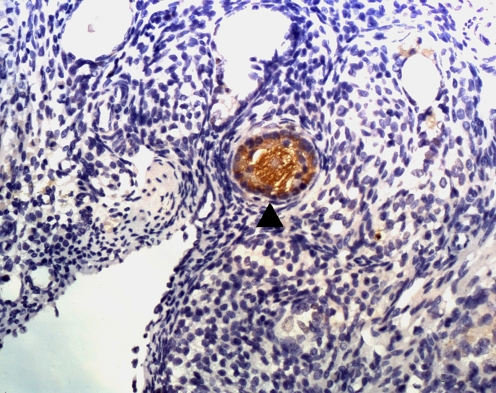
The percentage of follicles expressing Bax protein in Group A (fresh control group) was significantly lower than those in Group B (inclusion of caspase inhibitor) and Group C (non-inclusion of caspase inhibitor) (25.71 ± 5.12% versus 34.52 ± 7.51% and 43.87 ± 5.85%, P < 0.05). The percentage of follicles expressing Bax protein in Group B (inclusion of caspase inhibitor) was significantly higher than that in Group C (non-inclusion of caspase inhibitor) (34.52 ± 7.51% versus 43.87 ± 5.85%, P < 0.05) (Fig. 2).
Fig. 2.
The follicle expressing Bax protein (▲)
PCNA protein detection
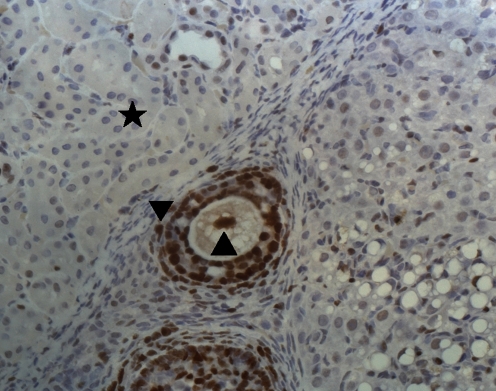
Grafted cortical strips demonstrated positive staining for Proliferating-cell nuclear (PCNA) expression in follicular and stromal cells. The percentage of follicular and stromal cells expressing PCNA in each image was calculated. The percentage of cells expressing PCNA in each image of grafts in Group A was significantly higher than those in Group B (inclusion of caspase inhibitor) and Group C (non-inclusion of caspase inhibitor) (11.21 ± 3.30% versus 7.81 ± 2.29% and 4.98 ± 1.62%, P < 0.05), and the percentage of cells expressing PCNA in each image of grafts in Group B (inclusion of caspase inhibitor) was significantly higher than that in Group C (non-inclusion of caspase inhibitor) (7.81 ± 2.29% versus 4.98 ± 1.62%, P < 0.05) (Fig. 3).
Fig. 3.
The follicular cell expressing PCNA in graft (▲), the stromal cell expressing PCNA in graft(▼), the kidney tissue (★)
Vaginal smear examination
The number of days before the resumption of estrous cycles of mice in Group A (fresh control group) was significantly less than those in Group B (inclusion of caspase inhibitor) and Group C (non-inclusion of caspase inhibitor) (7.15 ± 1.25 versus 11.21 ± 3.52 and 15.61 ± 4.70, P < 0.05 ), and the number of days before the resumption of estrous cycles of mice in Group B (inclusion of caspase inhibitor) was less than that in Group C (non-inclusion of caspase inhibitor) (11.21 ± 3.52 versus 15.61 ± 4.70, P < 0.05).
Discussion
With the significant increase in studies on cryopreservation of ovarian tissue conducted since 1990s, subsequent transplantation after completed cancer treatment has been suggested as an alternative to restore fertility for girls and women who are at high risk for ovarian failure after chemotherapy or radiotherapy [9]. Earlier work revealed that more than 50% of primordial follicles are lost as a result of the frozen-thawed process and initial ischemia [10, 11]. Although Mazur and Gao thought that the loss of follicles following cryopreservation is attributed to necrosis as a result of intracellular ice formation and osmotic stress [12, 13], most of studies suggested that a proportion of cells are lost by an apoptotic phenotype [14–16].
A previous study was designed to examine the effects of the anti-apoptotic agent sphingosine-1-phosphate (S-1-P) on ovarian cryopreservation efficiency, however, the results did not support S-1-P (20 umol) inclusion to improve tissue viability following cryopreservation [15]. Previous published examples of S-1-P,s ability to reduce follicular apoptosis have been in response to either chemo-or radiotherapy-induced apoptosis in marine ovarian tissue [17, 18]. Chemo-and radiotherapies are known to induce ceramide-activated apoptotic pathways [17–19], and therefore if cryopreservation does not activate the same apoptotic pathway, this would explain the lack of effect of S-1-P.
Activation of proteases of the caspase family appears to be one of the common mechanisms that are irreversibly connected to apoptotic cell death [20–23]. So far, 14 members of the caspase family are known, and they can roughly be classified as initiator and executioner caspases according to their function [24–26]. Caspase-3, which can be activated by the initiator caspase-8 and caspase-9, as well as by the serine protease granzyme B, is a well-characterized typical executioner caspase involved in many proteolytic processes in apoptotic cells [25, 26]. In particular, caspase-3 mediates the cleavage of proteins that are essential for cell stability, DNA repair and activation of Dnases [27–29]. Once cytosolic caspase-3 is activated, most cells are destined to die primarily by an apoptotic phenotype.
Several studies have reported that caspase 3 protein play a role in the apoptotic pathway of ovarian tissue induced by cryopreservation [14, 16]. The inhibition of caspase pharmacologically can block the completion of apoptosis [7]. It was reported that in cardiomyocytes, caspase inhibitors can reduce incidence of apoptosis induced by metabolic inhibition in the isolated rabbit heart [30]. In addition, previous studies revealed that the caspase inhibitors z-VAD-FMK and z-DEVD-fmk can reduced ischemic neuronal injury after cerebral ischemia [31, 32]. Therefore, we speculated that caspase inhibitors would be cryoprotective in the cryopreservation of ovarian tissue. The previous study demonstrated that a global caspase inhibitor, Z-VAD-FMK at the dose of 20 uM in vitrification and culture media could improve survival of vitrified porcine embryos[33], whereas the ovarian tissue has special characteristics: fixed geometry, high cell density, diversity of cell types and presence of a vascular system, therefore, the permeation of ovary with caspase inhibitor is much slower than that of isolation cells, so we added Z-VAD-FMK at the dose of 60 uM in cryoprotective solution in this study.
The results of TUNEL assay showed that the incidence of TUNEL positive follicles in Group B was significantly lower than that in Group C. The findings indicated that the globle caspase inhibitor Z-VAD-FMK can decrease apoptosis of ovarian tissue during the frozen-thawed process.
Regulation of apoptotic signaling is achieved by expression of distinct protein families, such as the bcl-2 family. When Bax is in excess and the homodimers of Bax dominate, cells are susceptible to programmed cell death [34]. It was reported that Bax protein was involved in the apoptotic pathway induced by cryopreservation, so the detection the expression of Bax protein can be used as a tool to evaluate the apoptosis induced by cryopreservation [14]. The percentage of follicles expression Bax protein in Group B was significantly lower than that in Group C. The results were consistent with those of TUNEL assay, thus confirmed the conclusion that Z-VAD-FMK prevent some of apoptosis of ovarian tissue induced by cryopreservation.
The day of initiating estrous cycles of mice in Group B was significantly less than that in Group C, we concluded that Z-VAD-FMK inclusion improve functional ability of grafts in this study.
Proliferating cell nuclear antigen (PCNA) is a protein involved in the cell cycle [35], and also serves as an auxiliary protein to DNA polymerase delta, which is involved in DNA synthesis and repair. It is possible that DNA polymerase delta might be activated to repaired possible damage to the genetic material in the oocytes selected to grow [36]. PCNA expression in follicular and stromal cells suggested that these follicles would be capable of further growth and development, and these stroma would be provide structure and support for developing follicles following grafting. A review of the literature concluded that this antibody can be used widely to assess the proliferative status of cell populations, furthermore, detection of the expression of PCNA has been used as a tool to evaluate the functional restoration of grafted ovary [15]. The data of present experiment revealed that the percentage of follicular and stromal cells expressing PCNA of grafts in Group B was significantly lower than that in Group C. We hypothesized that the protocol C should be more effective than protocol B in restoring endocrine function.
The global caspase inhibitor Z-VAD-FMK can decrease the apoptosis of ovarian tissue, thus relieve the cryo-induced damage of ovarian grafts, and the inclusion of a global caspase inhibitor (Z-VAD-FMK) in the cryopreservation media improves functional ability of grafts in this study. Nevertheless, further efforts are needed to be made to explore the optimal dose for caspase inhibitor in cryopreservation of ovarian tissue. These results shed light on efforts to optimize human ovary cryopreservation.
Acknowledgments
Appreciation is extended to Dr Bin Cai for his review and comment and Xiao-Hua Hu from department of pathology for supporting this work.
Conflict of interest statement The authors declare that there are no conflicts of interest.
Footnotes
Capsule Caspase inhibition is a valid therapeutic strategy in cryopreservation of ovarian tissue.
References
- 1.Baker TG. Radiosensitivity of mammalian oocytes with particular reference to the human female. Am J Obstet Gynecol. 1971;110:746–61. [DOI] [PubMed]
- 2.Apperley JF, Reddy N. Mechanism and management of treatment-related gonadal failure in recipients of high dose chemoradiotherapy. Blood Rev. 1995;9:93–116. [DOI] [PubMed]
- 3.Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286:1490–3. [DOI] [PubMed]
- 4.Baird DT, Webb R, Campbell BK, Harkness LM, Gsden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196°C. Endocrinology. 1999;140:462–71. [DOI] [PubMed]
- 5.Rimon E, Cohen T, Dantes A. Apoptosis in cryopreserved human ovarian tissue obtained from cancer patients: a tool for evaluating cryopreservation utility. Int J Oncol. 2005;27:345–53. [DOI] [PubMed]
- 6.Tirelli M, Basini G, Grasselli F, Bianco F, Tamanini C. Cryopreservation of pig granulosa cells: effect of FSH addition to freezing medium. Domest Anim Endocrinol. 2005;28:17–33. [DOI] [PubMed]
- 7.Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–70. [DOI] [PubMed]
- 8.Rugh R. The mouse: its reproduction and development. New York: Oxford University Press; 1990. p. 38–9.
- 9.Torrents E, Boiso I, Barri PN. Applications of ovarian tissue transplantation in experimental biology and medicine. Hum Reprod Update. 2003;9:471–81. [DOI] [PubMed]
- 10.Gosden RG. Low temperature storage and grafting of human ovarian tissue. Mol Cell Endocrinol. 2000;163:125–9. [DOI] [PubMed]
- 11.Nugent D, Neirow D, Brook PE, Aubard Y, Gosden RG. Transplantation in reproductive medicine: previous experience, present knowledge and future prospects. Hum Reprod Update. 1997;3:267–80. [DOI] [PubMed]
- 12.Gao D, Critser JK. Mechanisms of cryoinjury in living cells. ILAR. 2000;41:181–96. [DOI] [PubMed]
- 13.Mazur P. Freezing of living cells: mechanism and implications. Am J Physiol. 1984;247:C125–42. [DOI] [PubMed]
- 14.Fauqe P, Amor AB, Joanne CJ, Agnani G. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87:1200–7. [DOI] [PubMed]
- 15.Onions VJ, Mitchell MRP, Campbell BK, Webb R. Ovarian tissue viability following whole ovine ovary cryopreservation: assessing the effects of sphingosine-1-phosphate inclusion. Hum Reprod. 2008;23:606–18. [DOI] [PubMed]
- 16.Shin SY, Lee JY, Lee EY. Protective effect of vascular endothelial growth factor (VEGF) in frozen-thawed granulosa cells is mediated by inhibition of apoptosis. Eur J Obstet Gynecol Reprod Biol. 2006;125:233–8. [DOI] [PubMed]
- 17.Morita Y, Perez GI, Paris F. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–14. [DOI] [PubMed]
- 18.Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly TL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 1997;3:1228–32. [DOI] [PubMed]
- 19.Haimovitz-Friedman A, Kan CC, Ehleiter D, Persud RS, McLoughlin M, Fuks Z, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–35. [DOI] [PMC free article] [PubMed]
- 20.Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997;64:33–42. [DOI] [PubMed]
- 21.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. [DOI] [PubMed]
- 22.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–42. [DOI] [PubMed]
- 23.Thornberry N, Lazebnik Y. Caspase: enimies within. Science. 1998;281:1312–6. [DOI] [PubMed]
- 24.Earnshaw WC. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Bio Chem. 1999;68:383–424. [DOI] [PubMed]
- 25.Slee EA. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999;6:1074–87. [DOI] [PubMed]
- 26.Budihardjo I. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90. [DOI] [PubMed]
- 27.Liu X. DEF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–84. [DOI] [PubMed]
- 28.Simizu S. Requirement of caspase-3(-like)protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem. 1998;273:26900–7. [DOI] [PubMed]
- 29.Zheng T. Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc Natl Acad Sci USA. 1998;95:13618–23. [DOI] [PMC free article] [PubMed]
- 30.Gottlieb RA, Gruol DL, Zhu JY. Preconditioning rabbit cardiomyocytes: role of pH, vacuolar proton ATPase, and apoptosis. J Clin Invest. 1996;97:2391–8. [DOI] [PMC free article] [PubMed]
- 31.Himi T, Ishizaki Y, Murota SI. A caspase inhibitor blocks ischaemia-induced delayed neuronal death in the gerbil. Eur J Neurosci. 1998;10:777–81. [DOI] [PubMed]
- 32.Ma J, Endres M, Moskowiz MA. Synergistic effects of caspase inhibitors and MK-801 in brain injury after transient focal cerebral ischemia in mice. Br J Pharmacol. 1998;124:756–62. [DOI] [PMC free article] [PubMed]
- 33.Men H, Agca Y, Riley LK, Critser JK. Improved survival of vitrified porcine embryos after partial delipidation through chemically stimulated lipolysis and inhibition of apoptosis. Theriogenology 2006;2008–16. [DOI] [PubMed]
- 34.Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore: a cyclosporing-sensitive pore in inner mitochondrial membrane. J Bioenerg Biomembr. 1994;26:509–17. [DOI] [PubMed]
- 35.Xiong Y, Connolly T, Futcher B, Beach D. Human D-type cyclin. Cell. 1991;65:691–9. [DOI] [PubMed]
- 36.Liu Y, Marraccino RL, Keng PC. Requirement for proliferating cell nuclear antigen expression during stages of the Chinese hamster ovary cell cycle. Biochemistry. 1989;28:2967–74. [DOI] [PubMed]