Abstract
Diadenosine tetraphosphate (AP4A), two adenosine moieties bridged by four phosphates, is an endogenous purinergic ligand found in brain. Previous studies have shown that AP4A reduced neurodegeneration caused by the dopaminergic neurotoxin 6-hydroxydopamine in rat striatum and substantia nigra. The purpose of this study was to determine whether AP4A is protective against methamphetamine (MA) –mediated toxicity. Primary neuronal cultures were prepared from rat embryonic (E14- E15) ventral mesencephalic tissue. Cultures treated with 2 mM MA exhibited decreased tyrosine hydroxylase (TH) immunoreactivity and increased cleaved caspase-3 immunoreactivity and TUNEL labeling. All these changes were lessened by pretreatment with AP4A. The protective effect of AP4A was also found in vivo. Adult Sprague-Dawley rats were injected with AP4A (25 μg/ 20 μl) or vehicle intracerebroventricularly followed by 4 doses of MA (5 or 10 mg/ kg), given subcutaneously every two hours. Administration of MA reduced locomotor activity one day after injection, which was significantly antagonized by the pretreatment with AP4A. Using immunohistochemical analysis, TH fiber density at the substantia nigra pars reticulata was found reduced while cleaved caspase-3 immunoreactivity in striatum was increased after MA treatment; these responses were also significantly antagonized by AP4A. Taken together, our data show that AP4A has protective effects against MA-mediated toxicity both in vitro and in vivo. The mechanism of action involves suppression of MA -induced apoptosis.
Keywords: Diadenosine tetraphosphate, methamphetamine, apoptosis, neuroprotection, dopamine
Introduction
Diadenosine tetraphosphate (AP4A) is a compound that contains two adenosine moieties bridged by 4 phosphates. AP4A is found in tears (Pintor et al., 2002), heart, and brain (Emanuelli et al., 1998; Kisselev et al., 1998; Oaknin et al., 2001). Oxidative stress induces synthesis of AP4A (Bochner et al., 1984). Application of AP4A suppressed hypoxia -induced TUNEL labeling in primary cortical cultures (Wang et al., 2003). In vivo, AP4A reduced translocation of mitochondrial cytochrome C, activation of cytoplasmic caspase-3, and cerebral infarction in ischemic cerebral cortex in vivo (Wang et al., 2003). These data suggest that AP4A is protective, via anti-apoptotic mechanisms, against ischemic injury in cerebral cortex.
AP4A also has positive effects on dopaminergic neurons. Selective AP4A binding sites were found in substantia nigra and striatum (Oaknin et al., 2001; Pintor & Miras-Portugal, 1995). Pretreatment with AP4A antagonized 6-hydroxydopamine (6-OHDA) – mediated neurodegeneration, including motor bias, and decreased tyrosine hydroxylase (TH) immunoreactivity in substantia nigra and striatum. AP4A also attenuated the 6-OHDA-mediated reduction of dopamine release in the striatum. These findings suggest that AP4A is capable of protecting dopaminergic neurons from the toxic effects of 6-OHDA (Wang et al., 2003). Interestingly, administration of amphetamine causes release of endogenous stores of AP4A in the striatum (Pintor et al., 1995; Pintor et al., 1993). The protective role of AP4A against amphetamine analogs has not been characterized.
Methamphetamine (MA) is a commonly abused drug worldwide. Neurotoxicity of MA has been reported in dopaminergic and non-dopaminergic cells in various brain regions including cortex, striatum and hippocampus (Deng et al., 2001; Jayanthi et al., 2004; Schmued & Bowyer, 1997; Zhu et al., 2006). Several mechanisms of MA-induced toxicity have been identified including oxidative stress, excitotoxicity and apoptosis (Cadet et al., 2007). In rodents, acute administration of high doses of MA, in vivo or in vitro, activates caspase-3 and poly(ADP-ribose) synthetase (PARS), upregulates p53, and results in DNA fragmentation and cell death in neurons, indicating apoptosis as a primary mechanism (Cadet et al., 2003). Since AP4A has protective effects against 6-OHDA injury in dopaminergic neurons (Wang et al., 2003), we examined the protective effects of AP4A against MA-induced toxicity in the CNS. We found that AP4A reduced MA –mediated toxicity both in vivo and in vitro.
Materials and Methods
Primary cultures of rat ventral mesencephalon
Primary cultures were prepared from embryonic (E14-15) ventral mesencephalon (VM) tissues obtained from fetuses of timed-pregnant Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), according to published procedures with some modification. The whole brain was removed aseptically and a small piece of tissue comprising the VM was dissected. After removing the blood vessels and meninges, pooled VM tissues were trypsinized (×1; Invitrogen, Carlsbad, CA) with gentle mixing at 5 minute intervals for 20 min. After rinsing off trypsin with ice – cold DMEM/F-12 (Invitrogen), cells were dissociated by trituration, counted and plated into 48 (12 × 104/well) or 96-well (6.0 × 104/well) cell culture plates pre-coated with poly-lysine (Becton-Dickinson, Franklin Lakes, NJ). The culture plating medium consisted of Dulbecco's modified Eagle medium/F12 supplemented with 10% heat-inactivated fetal bovine serum, 1 mM L-glutamine and 2% B27 (Invitrogen). Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The cultures were fed by exchanging 50% of media with feed media (Neurobasal medium (Invitrogen) with 2% B27 supplement and 0.5 mM L-glutamine) on DIV (days in vitro) 2 and 5. ON DIV7, cultures were fed with feed media containing B27 supplement without antioxidants (Invitrogen). AP4A (Sigma, St. Louis, MO) was given as 50% media exchange on DIV 9. MA (methamphetamine HCl, St. Louis, MO) was added as an 11× concentrate at 10-15 minutes after administration of MA. Cells were returned to a 37°C incubator for 2 days then fixed with 4 % paraformaldehyde (PFA) for immunoreactivity.
In vitro immunoreactivity and quantitation
After removing PFA solution, cells were washed with PBS and the fixed cultures were treated for 1 hour with blocking solution (2% BSA, 0.01% Triton X-100 and 5% goat serum in PBS). The cells were then incubated for 2 days at 4 °C with a mouse monoclonal antibody against TH (1:500; Chemicon, Temecula, CA, USA), Activated caspase-3 (rabbit anti-cleaved caspase-3 1:100, Cell Signaling Technology, Beverly, MA) or GFAP (mouse anti-GFAP, 1:500, Chemicon, Temecula, CA, USA). The cells were then rinsed three times in PBS. The bound primary antibody was visualized using the AlexaFluor 488 goat anti-mouse or AlexaFluor 568 goat anti-rabbit secondary (Molecular Probes). Images were acquired using a SPOT RT camera (Diagnostic Instruments, Inc., Sterling Heights, MI) attached to a NIKON TE2000 inverted microscope. TH+ or TUNEL+ cells were manually counted in 4× images acquired from 96-well plates using Metamorph Software (Molecular Devices, Sunnyvale, CA). Activated-caspase-3+ cells were manually counted from 10× images (4 fields per well of 96 well plate). The GFAP+ fibers were quantified from 10× images (4 fields per well of 96 well plate) acquired using the same camera settings. Pixels representing GFAP+ fibers were measured using Metamorph software at identical threshold settings. All immunoreactive counts and quantitation were expressed as percentage of untreated cells. Experiments were repeated 2-3 times with n=3-9 wells per group per experiment.
In vitro Terminal deoxynucleotidyl transferase (TdT)-mediated dNTP nick -end labeling (TUNEL)
Cultures were assayed for DNA fragmentation using a TUNEL-based method (In Situ Cell Death Detection Kit; Roche, Indianapolis, IN). Briefly, 4% PFA fixed cells were permeabilized in 0.5% Triton X-100 in 0.01 M PBS for 5 min on ice. To label damaged nuclei, 50 μL of the TUNEL reaction mixture was added to each sample and kept at 37 °C in a humidified chamber for 60 min. Procedures for positive and negative controls were carried out as described in the manufacturer's manual (Roche). Controls consisted of not adding the label solution (terminal deoxynucleotidyl transferase) to the TUNEL reaction mixture or pre-digesting slides with Dnase I. Material was examined using a Nikon TE2000 inverted microscope equipped with fluorescence.
Animals
Adult male Sprague Dawley rats from Charles River Lab Inc. were used for this study. Animals were anesthetized with chloral hydrate (400 m/kg, i.p.). AP4A (25 μg/ 20 μl) or vehicle (20 μl, 0.9% NaCl, Hospira, Inc, Lake Forest, IL) was injected through a Hamilton syringe into the lateral ventricle (coordination: AP: -0.8mm; Lat: 1.5mm; DV: 3.7mm). Four doses of MA (5 or 10 mg/ kg, s.c.) or saline (0.1 ml/100g body weight, s.c.) were given every 2 hours starting from 10 to 15 minutes after i.c.v injection. During MA or saline injections, animals were singly housed at room temperature (25°C) without bedding to prevent aspiration. Following these injections, animals were returned to group housing (2 animals per cage) with access to food and water ad libitum.
Behavioral measurements
Rats were placed in an Accuscan activity monitor (Columbus, OH) at 1-day after MA or saline injections. The monitor contained 16 horizontal and 8 vertical infrared sensors spaced 2.5 cm apart. The vertical sensors were situated 10 cm from the floor of the chamber. Each animal was individually placed in a 42×42×31 cm plexiglass open box. Motor activity was calculated using the number of beams broken by the animals.
Immunostaining rat brain sections for activated caspase-3 and tyrosine hydroxylase (TH)
Animals were euthanized at 2 or 4 days after MA injections for activated caspase-3 (Jayanthi et al., 2004)or tyrosine hydroxylase (Chou et al., 2008) immunoreactivity. Animals were anesthetized with chloral hydrate (400 mg/ kg i.p.) and perfused transcardially with saline followed by 4% paraformaldehyde in phosphate buffer (PB; 0.1 M; pH 7.2). The brains were dissected, post-fixed in PFA for 16 hours, and transferred to 18% sucrose in 0.1 M PB for at least 16 hours. Serial sections of the entire brain were cut at 25 μm thickness using a cryostat (Leica, Bannockburn, IL). One series of every sixth section was used for immunoreactivity. In order to control for staining variability, specimens from all experimental groups were included in every batch and reacted together in a net well tray under the same conditions. Sections were rinsed in 0.1M phosphate buffer, blocked with 4% bovine serum albumin (BSA) and 0.3% Triton x-100 in 0.1M PB. Sections were then incubated in a primary antibody solution, rabbit polyclonal anti-caspase-3 (Cell Signaling Technology, Beverly, MA, Asp175, 1:100) or mouse anti-TH (Chemicon, Temecula, CA., 1:250) diluted in 4% BSA and 0.3% Triton x-100 in 0.1M PB, concentration for 17-19 hours at 4 °C. Sections were rinsed in 0.1M PB and incubated in biotinylated goat anti-rabbit IgG (1:200; Vector Laboratories, Burlingame, CA) or horse anti-mouse IgG (1:200; Vector Laboratories, Burlingame CA) for 1 hour, followed by incubation for 1 hour with avidin-biotin-horseradish peroxidase complex. Staining was developed with 2,3′ diaminobenzidine tetrahydrochloride (0.5 mg/mL). Control sections were incubated without primary antibody. Sections were then mounted on slides and cover-slipped.
Analysis of histological images
The optical density of TH immunoreactivity in striatum was analyzed using Scion Image (ver 4.02) and averaged from 3 sections with a visualized anterior commissure (AP:-0.26 mm, -0.4 mm, -0.8 mm to bregma). TH fiber optical density in substantia nigra pars reticulata (SNpr) and TH neuronal density in substantia nigra pars compacta (SNpc) were quantified using Metamorph software (Molecular Devices, Downingtown, PA) and averaged from 3 sections (AP:-5.8 mm, -6.04 mm, -6.3 mm to bregma). TH optical density and TH neuron counts from right and left hemispheres were averaged in each animal for statistical analysis.
Using Metamorph software (Molecular Devices), the number of caspase-3 (+) cells was counted in 2 striatal regions near the i.c.v. injection tract in each brain (AP: -0.8 mm to bregma). The presence of lateral and 3rd ventricle, fornix and triangular septal nuclei as well as the appearance of the corpus callosum were used as anatomical landmarks to obtain similar sections for imaging. Average size of caspase-3(+) cells was determined by Metamorph software to be 175 μm2. All measurements were done by blinded observers.
In vivo microdialysis
Animals were anesthetized with chloral hydrate (400mg/ kg, i.p.). Dialysis guide cannulae (20 gauge, 14 mm) were implanted over the striatum (AP: 0.0 mm, Lat 2.5 mm to the bregma and DV −4.0 mm to brain surface). The guide cannulae were fixed to the skull with four stainless steel screws and dental acrylic. Dialysis experiments began 1 week after the surgical procedure. Dialysis probes were constructed as previously described with some modifications. The active region of the dialysis membrane was 0.8-1.5 mm in length. Probes were inserted into the striatum at least 12 hr before perfusion to minimize the effects of surgery-induced dopamine release during the experiment. On the day of the experiment, dialysis buffer (5 mM glucose, 2.5 mM KCl, 140 mM NaCl, 1.4 mM CaCl2, 1.2 mM MgCl2, and 0.15% PBS, pH 7.4) was perfused through the probe (2.0 μl/min) for at least 2 hr before sample collection. Dialysis samples were collected every 20 min into 10 μl of mobile phase.
Quantification of dopamine by HPLC
For the measurement of extracellular dopamine, samples were collected into 10 μl of mobile phase (4.76 mM citric acid, 150 mM NaH2PO4, 50 μM EDTA, 3 mM SDS, 10% methanol (v/v), and 15% acetylnitrile (v/v), pH 5.6. All samples were frozen at −80 °C until analysis. The samples were subsequently thawed and placed in an ESA model 540 autosampler connected to an HPLC system with electrochemical detection. Dopamine was separated using a 150 ×3.2mm C18 reversed-phase column (ESA Laboratories, Inc., Chelmsford, MA) and oxidized-reduced using coulometric detection (ESA Laboratories). Three electrodes were used: a preinjection port guard cell (+0.25 V) to oxidize the mobile phase, a reduction analytical electrode (E1, −0.1 V), and an oxidation analytical electrode (E2, +0.2 V). The area under the curve of dopamine peak was measured with an ESA 501 Chromatography Data System. Dopamine values were normalized to the internal standard dihydroxybenzylamine (100 pg/ 10 μL) and compared with an external standard curve for quantification.
Results
Neuroprotection in cell culture
a) TH and GFAP immunoreactivity
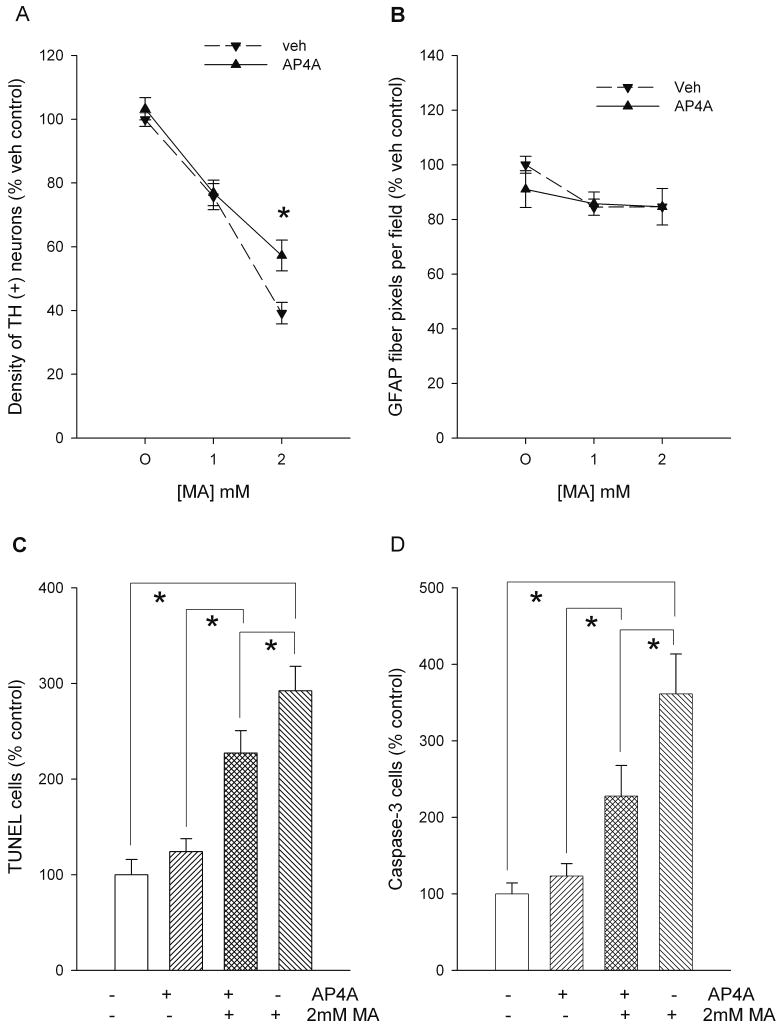
The survival of dopaminergic neurons in VM cultures was examined using TH immunoreactivity. Two-day exposure to MA dose-dependently decreased the density of TH (+) cells (p<0.001, F(2,140)= 106.019, 2-way ANOVA, Figures 1A and 2). AP4A (100 μM) significantly antagonized the decrease in TH (+) cells caused by 2 mM MA (p=0.048, F(2,140)= 3.100, 2-way ANOVA, Figures 1A and 2).
Figure 1.
AP4A reduces methamphetamine (MA) toxicity in primary VM cultures. (A) Density of TH (+) cells. MA decreased the density of TH (+) neurons. AP4A (100 μM) antagonized the decrease in TH (+) cell density induced by 2 mM MA. (B) Density of GFAP immunoreactivity. AP4A did not alter GFAP immunoreactivity in cultures treated with MA. (C) TUNEL labeling. Pretreatment with AP4A reduced MA -mediated TUNEL labeling. (D) Activation of caspase-3. Density of activated caspase-3 positive cells was enhanced by MA, which was significantly reduced by AP4A. All data were normalized to the mean of vehicle/no MA controls in each experiment. * p<0.05, 1- or 2-Way ANOVA.
Figure 2.
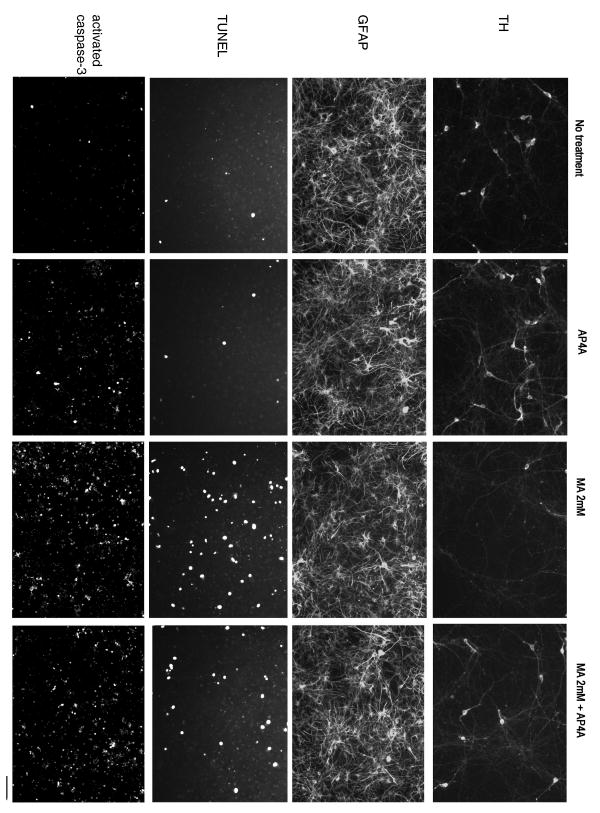
Photomicrographs of culture VM cells treated with MA and AP4A. MA reduced the density of TH (+), and increased the density of TUNEL (+) and activated caspase-3 cells. Pretreatment with AP4A antagonized these MA –induced changes. AP4A and MA did not alter GFAP immunoreactivity. Calibration: TH, GFAP, TUNEL: 80 μm; activated caspase 3: 160 μm.
Treatment with MA slightly decreased the density of nuclei, as measured by 4′6-diamidino-2-phenylindole dihydrochloride (DAPI) staining although the decreases did not reach statistical significance (p=0.061, F(2,53)= 2.932, 1-way ANOVA). The interaction of MA and AP4A on glia was examined by GFAP immunoreactivity. Neither MA (F(1,12)=0.247, p=0.628, 2 way ANOVA) nor AP4A (F(1,12)=3.084, p=0.105, 2-way ANOVA) significantly changed GFAP immunoreactivity (Fig 1B and 2).
b) TUNEL and caspase-3 activity
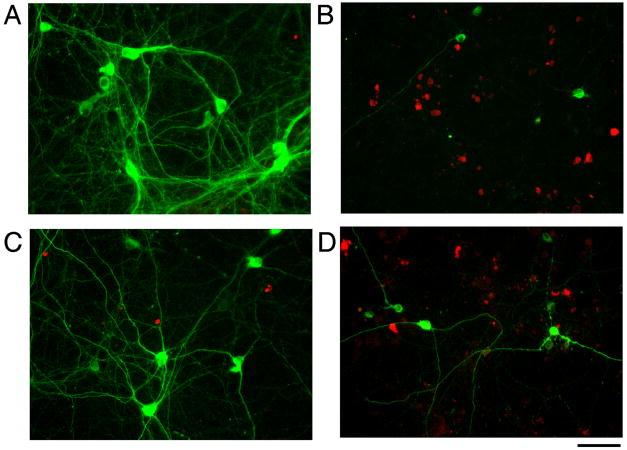
Previous studies have indicated that MA causes apoptosis in vitro. We found that 2 mM MA increased TUNEL labeling (Fig 1C & 2, p<0.05, F(3,56)=19.817, 1-Way ANOVA+ Newman-Keuls test). AP4A significantly reduced MA-mediated TUNEL labeling (Fig 1C & 2, p<0.05, F(3,56)=19.817, 1-Way ANOVA+ Newman-Keuls test). Activated caspase-3 immunoreactivity was used as another marker of apoptosis. Exposure to MA increased the density of activated caspase-3 positive cells and this increase was reduced by AP4A (Figures 1D & 2; p<0.05, F(3,48)=11.827, 1-way ANOVA+ Newman-Keuls test). Using a double immunoreactivity technique, we found that only few TH cells were immunoreactive for activated caspase-3 (Fig 3). Neither AP4A nor MA significantly altered the density of cells co-labeled with TH and caspase-3.
Figure 3.
MA decreased TH-immmunoreactivity and increased activated caspase-3 immunoreactivity in VM cell culture. Red: activated caspase-3 cells, green: TH cells. MA reduced TH, but increased activated caspase-3 immunoreactivity (A: vehicle; B: MA). There is little colocalization of TH and activated caspase-3. (C) AP4A alone did not alter caspase-3 expression, but (D) reduced the density of activated caspase-3 positive cells in the presence of MA. Calibration= 80 μm.
Neuroprotection in vivo
a) Locomotor activity
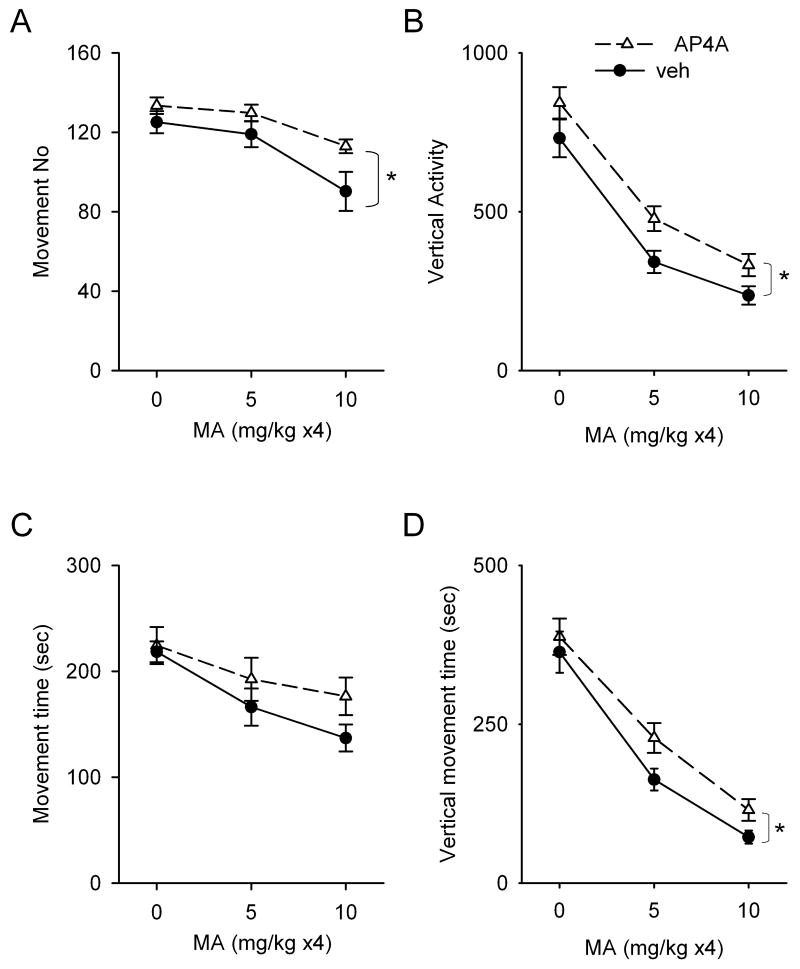
A total of 52 rats were used to examine the locomotor activity at one day after parenteral saline or MA injection. These animals were divided into 3 groups as follows. (A) Twenty rats were injected with either AP4A (n=10) or vehicle (n=10) and then 4 doses of saline. (B) Sixteen rats were treated with either AP4A (n=8) or vehicle (n=8) and then 4 doses (5mg/ kg) of MA. (C) Sixteen rats were treated with either AP4A (n=8) or vehicle (n=8) and then 4 doses (10mg/ kg) of MA. Locomotor activity was monitored continuously for 30 min and reported in three 10-min intervals at one day after the first dose of MA or saline. In the animals receiving systemic saline injection, there is a time dependent decrease in motor activity. Movement number, horizontal movement time, vertical activity, and vertical movement time peaked in the first 10-min exploring interval and then significantly decreased in the 2nd and 3rd 10-min periods. MA, at doses of 5 mg/ kg ×4 or 10 mg/ kg ×4, significantly reduced locomotor activity to 40 to 60% of control in both the initial 10-min (Fig 4 A-D, p<0.001, 2-way ANOVA) and subsequent phases. In the first 10-min interval (Fig 4 A-D), intracerebroventricular administration of AP4A did not alter locomotor activity in animals receiving saline; however, AP4A significantly enhanced horizontal movement number (Fig 4A, p=0.006, F(1,46)=8.392, 2-way ANOVA), vertical activity (Fig 4B, p=0.003, F(1,46)=9.599, 2-way ANOVA), and vertical movement time (Fig 4D, p=0.033, F(1,46)=4.818, 2-way ANOVA) in MA –treated rats. A marginal increase in horizontal movement time was also found in animals treated with AP4A (Fig 4C, p=0.078, F(1,46)=3.241, 2-way ANOVA). In the 2nd and 3rd 10-min intervals, AP4A did not alter MA –induced bradykinesia (p>0.05, two way ANOVA).
Figure 4.
Interactions of AP4A and MA in the first 10-min exploratory phase of locomotor activity. Locomotor parameters were monitored at one day after systemic MA or saline injection in adult rats. In animals receiving i.c.v. vehicle pretreatment, MA dose-dependently reduced (A) movement number, (B) vertical activity, (C) horizontal movement time, and (D) vertical movement time. Pretreatment with AP4A significantly enhanced movement number, vertical activity and vertical movement in MA –treated rats. A marginal increase in horizontal movement time was found in animals treated with AP4A (p=0.078, 2-way ANOVA).
b) TH immunoreactivity (THir)
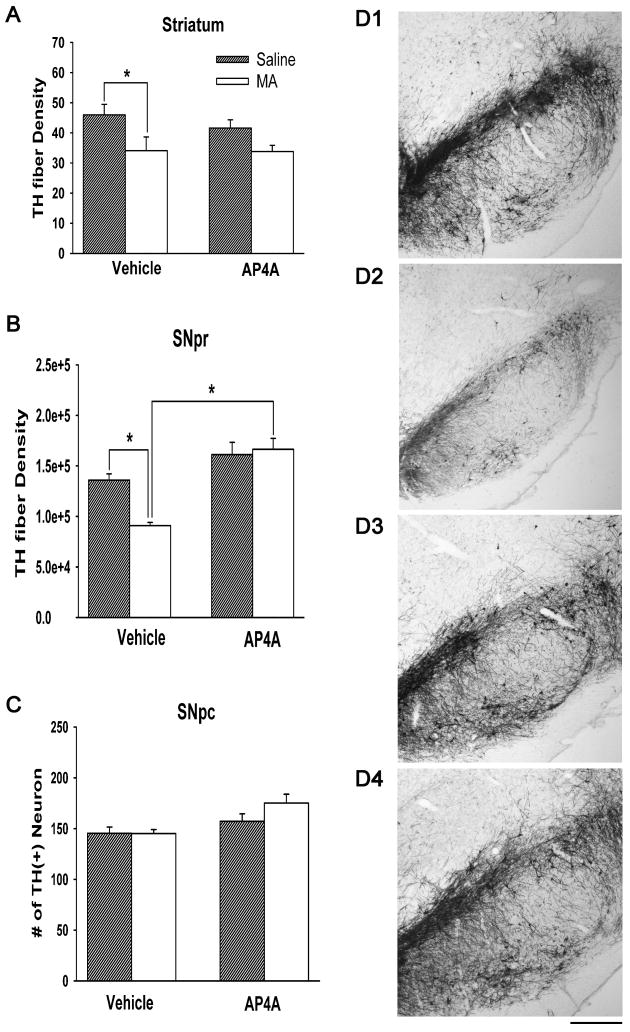
Twenty-nine rats were used for THir analysis at 4 days after injection. Of these, 7 were treated with veh (i.c.v.) and then saline (s.c.), 7 were treated with veh (i.c.v.) and then MA (10 mg/ kg ×4, s.c.), 8 were treated with AP4A (i.c.v.) and then saline (s.c.), and 7 were treated with AP4A (i.c.v.) and then MA (s.c.). THir was analyzed in 3 regions: THir optical density in striatum; THir fiber density in SNpr; and THir neuron density in SNpc. In striatum (Fig 5A), administration of AP4A did not alter THir density (p=0.491, F(1,25)=0.489, two way ANOVA,). Parenteral administration of MA significantly reduced striatal THir fiber density (p=0.006, F(1,25)=8.850, 2-Way ANOVA). Administration of AP4A did not significantly reduce the loss of THir fiber density in striatum in MA –treated rats (p=0.537, F(1,25)=0.391, 2-Way ANOVA). In SNpc (Fig 5C), MA did not alter the density of TH neurons (p=0.207, F(1,25)=1.678, 2-Way ANOVA). There was no significant interaction in TH neuronal density between treatments with AP4A and MA (p=0.197, F(1,25)=1.760, 2-Way ANOVA). In SNpr (Fig 5B), MA administration significantly reduced THir density (p=0.040, F(1,25)=4.700, 2-Way ANOVA). There is a significant interaction in THir between treatments with AP4A and MA (p=0.011, F(1,25)=7.485, 2-Way ANOVA). Post-hoc Newman-Keuls analysis indicates that AP4A significantly increased THir in SNpr in animals receiving MA (p<0.001). Typical immunostaining of TH in SNpr is demonstrated in Fig 5D (D1: vehicle +saline; D2: vehicle +MA; D3: AP4A +saline; D4: AP4A +MA). These histological findings suggest that intracerebroventricular administration of AP4A has protective effects on the TH terminals in SNpr, reducing MA toxicity.
Fig 5. TH immunoactivity in striatum, SNpc, and SNpr.
Animals were treated with veh or AP4A (i.c.v.) and then saline or MA (s.c.). THir was analyzed 4 days after injection. (A) In striatum, parenteral administration of MA significantly reduced density of striatal THir. Administration of AP4A did not significantly reduce the loss of THir density in MA – treated rats. (B) In SNpr, MA administration significantly reduced THir density. AP4A significantly increased THir in SNpr in animals receiving MA. (C) In SNpc, MA did not alter the density of TH neurons. There is no significant interaction in TH neuronal density between treatments with AP4A and MA. (D) Typical immunostaining in SNpr. (D1: vehicle+saline; D2: vehicle+MA; D3: AP4A +saline; D4: AP4A +MA). Calibration = 500 μm.
c) Activated Caspase-3 immunoreactivity in vivo
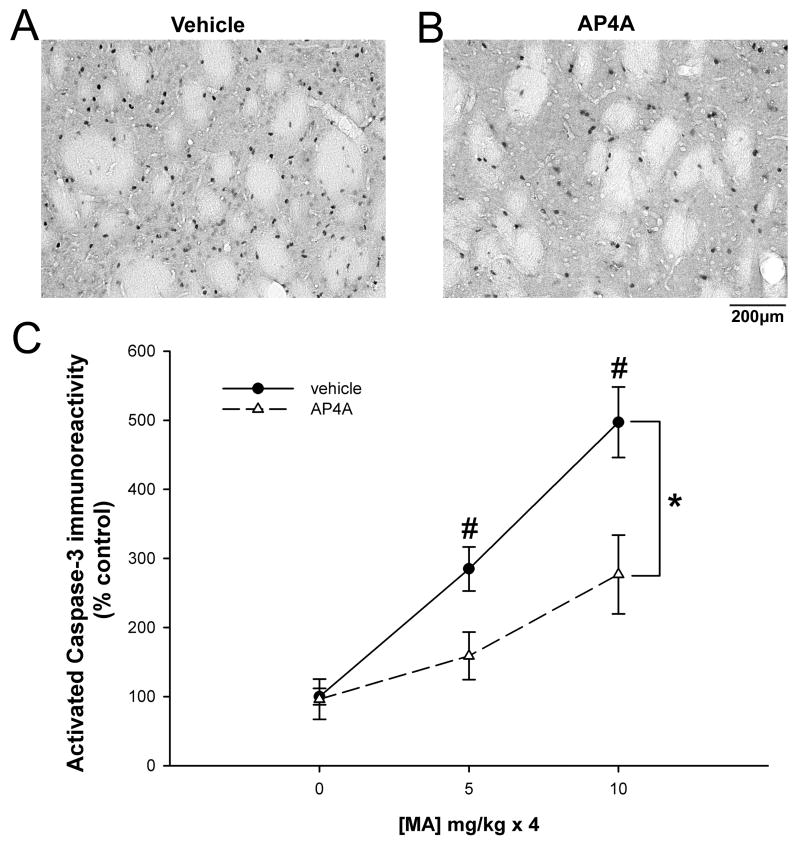
A total of 34 rats were used for caspase-3 immunoreactivity. Eighteen animals received AP4A (25 μg/ 20 μl, i.c.v.) and another 16 rats were injected with vehicle (saline, 20 μl, i.c.v.). Animals were treated with saline (10 mg/ kg ×4, n=11), low (5 mg/ kg ×4, n=12) or high (10 mg/ kg × 4, n=11) doses of MA and killed for activated caspase-3 immunoreactivity 2 days later. The number of activated caspase-3 (+) cells was imaged bilaterally in the striatum from anatomically equivalent sections (AP: -0.8 mm from bregma) for each brain (Figure 6). MA increased activated caspase-3 immunoreactivity in striatum in a dose-dependent manner (Figure 6C; p<0.001, F(2,,28)=26.284, 2-way ANOVA). Pretreatment with AP4A significantly antagonized MA –mediated caspase-3 activation (Figure 6A, vehicle vs. 6B, AP4A; Figure 6C, p<0.001, F(1, 28)=13.178, 2-way ANOVA). There is a statistically significant interaction between the effect of AP4A and the dose of MA (p=0.038, F(2,28)=3.686, 2-Way ANOVA). Post-hoc Newman-Keuls analysis indicates that striatal caspase-3 immunoreactivity induced by MA at either 5 mg/ kg × 4 or 10 mg/ kg × 4 was significantly reduced by AP4A (p<0.05).
Figure 6.
MA- induced caspase-3 activation in rat striatum is suppressed by AP4A. Animals were injected with AP4A or vehicle into the left lateral cerebral ventricle followed by systemic administration of MA. Activated caspase-3 immunoreactivity was analyzed 2 days after MA injection. Examples of striatal immunoreactivity for activated caspase 3 in MA-injected pretreated with vehicle (A) or AP4A (B). MA increased activated caspase-3 immunoreactivity in striatum in a dose-dependent manner (p<0.001, 2-way ANOVA). (C) Pretreatment with AP4A significantly antagonized MA – mediated caspase-3 activation (*p<0.001, 2-way ANOVA). AP4A significantly reduced striatal caspase-3 immunoreactivity induced by MA at 4×5 mg/ kg or 4×10 mg/ kg (#p<0.05, 2-way ANOVA, SNK post-hoc analysis).
d) Dopamine release from striatum
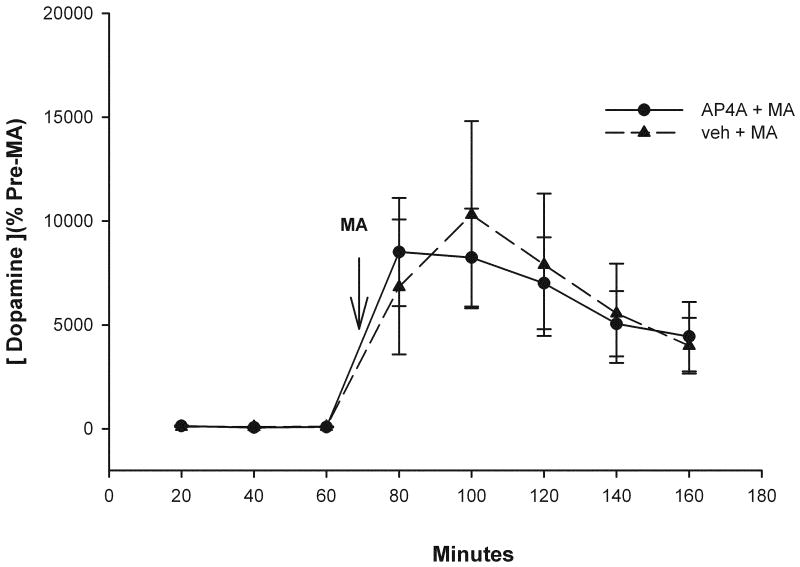
Basal and MA-evoked dopamine release from dorsal striatum was measured using microdialysis and HPLC techniques in 12 rats. Six animals were perfused with AP4A (25 μg/μl, 2 μl/min) and another 6 rats were perfused with vehicle. Administration of AP4A did not alter basal dopamine release (data not shown, p>0.05, F(5,30)=2.080, 1-way ANOVA). Systemic injection of MA (10 mg/ kg, s.c.) induced dopamine overflow (p<0.05, F(1,92)=32.628, 2-way ANOVA) which lasted for more than 100 min (Figure 7). AP4A did not alter the peak or the duration of dopamine overflow induced by MA (Figure 7, p=0.902, F(1,92)=0.0154, 2-Way ANOVA).
Figure 7.
AP4A did not alter basal and MA -evoked dopamine release from dorsal striatum. Animals were perfused with AP4A (25 μg/μl, 2 μl/min) or vehicle through the microdialysis cannula. Systemic injection of MA at 10 mg/ kg, (arrow) induced dopamine overflow (dashed line). The presence of AP4A did not alter the peak or the duration of dopamine overflow induced by MA (solid line).
Discussion
In this study, we found that density of TH (+) neurons and fibers in primary VM cultures were significantly reduced 2 days after MA application. GFAP (+) cells were not affected by MA. These data suggest that TH neurons are sensitive to the toxic effects of MA and the effects of MA were not due to non-specific effects causing overall cell loss in vitro. High doses (≥ 1mM in vitro or ≥ 5mg/ kg in vivo) of MA are required to produce caspase-3 activation and DNA fragmentation. These data are in agreement with previous studies (Deng et al., 2001; Chou et al., 2008b; Jayanthi et al., 2005) and suggest that acute administration of high doses of MA caused activation of apoptotic pathways that lead to the death of VM neurons including dopaminergic neurons.
AP4A has been shown to reduce 6-OHDA -mediated injury in VM cells (Wang et al., 2003). Since 6-OHDA shares several neurodegenerative mechanisms with MA in DA neurons, such as apoptosis (Ali et al., 1996), it is possible that AP4A can reduce MA toxicity by mechanisms similar to that seen with 6-OHDA. In this study, we found that pretreatment with AP4A attenuated the MA –mediated decrease in the density of THir fiber density in VM cultures, suggesting that AP4A is neuroprotective in VM cells.
The protective effect of AP4A was also found in vivo. Previous studies have indicated that dopamine plays an important role in exploring activity. For example, blockade of dopaminergic receptors abolished the detection of spatial novelty in rodents (Roullet et al., 1996). We found that MA dose- dependently reduced exploratory behavior one day after injection. Horizontal movement number, vertical movement time, and vertical activity over the first 10 min were all significantly reduced after high doses of MA. Pretreatment with AP4A attenuated MA-induced decrease in exploratory locomotor behavior, which may be attributed to the histological changes in dopaminergic neurons. Further studies are needed to determine the mechanisms of AP4A on exploratory behavior. Overall, these data suggest that pretreatment with AP4A can lessen behavioral deficits induced by high doses of MA.
Similar to previous reports, we also found that high doses of MA reduced TH immunoreactivity in striatum and SNpr (Chou et al., 2008b; Chou et al., 2008a). There was less influence on the TH neuronal density in the SNpc. Administration of AP4A did not alter the reduction of TH fiber density in striatum in MA –treated rats. However, AP4A reduced the MA-mediated decrease in THir in SNpr. Since dendritic DA release and receptor activation in SNpr play important roles in regulating motor behavior (Radnikow & Misgeld, 1998; Timmerman & Abercrombie, 1996), the AP4A -mediated change in THir in SNpr seen in this study may contribute to the behavioral normalization after MA injection. Taken together, these histological and behavioral data suggest AP4A, given exogenously, can reduce MA –mediated toxicity in dopaminergic circuits in vivo.
There is increasing evidence indicating that MA induces degeneration through activation of apoptosis (Cadet et al., 2007). The anti-apoptotic effects of AP4A were initially observed in a rodent model of ischemia (Wang et al., 2003). Pretreatment with AP4A reduced cytochrome C translocation, activation of caspase-3, and TUNEL labeling after ischemic injury in cortical neurons, suggesting that AP4A can suppress programmed cell death in stroke animals (Wang et al., 2003). In the present study, we further explored the anti-apoptotic effect of AP4A after MA insults. AP4A antagonized MA – elicited TUNEL labeling and activation of caspase-3, markers of apoptosis, in VM cells in vitro. These data suggest that the protective effect of AP4A against MA –induced toxicity in VM culture is mediated through anti-apoptotic mechanisms.
Although MA induces neurodegeneration in TH neurons, the activation of capsase-3 was found mostly in non-TH (+) cells in culture. These data suggest that MA may lesion the dopamine as well as non-dopaminergic cells in culture. This possibility is consistent with previous reports that similar doses of MA can kill non-dopaminergic neurons in primary cortical cells (Stumm et al., 1999), immortalized mesencephalic cells (Cadet et al., 1997), as well as immortalized striatal cells (Deng et al., 2002). It is also possible that the injured TH cells that possessed activated caspase-3 activity may lose their TH expression by 2 days or that caspase-3 activation in TH neurons may take place earlier than 2 days. These possibilities require further testing in future studies.
The anti-apoptotic effects of AP4A were also demonstrated in vivo. We found that repeated administration of MA, at 5 or 10 mg/ kg ×4, increased activated caspase-3 immunoreactivity in the striatum, which was reduced by pretreatment with AP4A. Taken together, our data suggest that the protective effects of AP4A against MA toxicity are mediated through the suppression of apoptosis both in vivo and in vitro.
There are other mechanisms that may contribute to AP4A-induced protection. Hyperthermia plays an important role in MA toxicity (Bowyer et al., 1994) and lowering body temperature attenuates MA –induced depletion of striatal dopamine (Ali et al., 1996; Bowyer et al., 1992). In this study, we found that AP4A did not attenuate MA -induced hyperthermia (data not shown). Because AP4A does not alter blood gases, blood pressure, and cerebral blood flow (Wang et al., 2003), it is not likely that AP4A – mediated protection against MA is due to changes in these physiological parameters.
MA can induce dopamine overflow in the striatum by increasing dopamine release, and inhibiting its uptake and metabolism by monoamine oxidase. The excess dopamine generates reactive quinones (Emdadul et al., 2003) which can damage both dopaminergic and non-dopaminergic cells. Using microdialysis, we found that AP4A did not alter the MA-induced increases in extracellular DA in the rat striatum. This observation indicates that the protective effects of AP4A are not the result of altering MA-induced DA release.
Previous studies have indicated that systemic administration of amphetamine (5 mg/kg) can release AP4A from caudate putamen up to 80 min in rats (Pintor et al., 1993); this effect is blocked by haloperidol (Pintor et al., 1995). These data suggest that amphetamine -induced AP4A release is indirectly mediated through DA receptors. Since MA is structurally similar to amphetamine, and furthermore, also increases extracellular DA concentration, it is likely that MA can release AP4A through a similar DA receptor –mediated mechanism. In this study, we found that AP4A reduced MA –mediated damage without affecting dopamine release, it is possible that endogenous AP4A might serve as a co-released protectant against DA-induced oxidative stress.
In summary, our data suggest that AP4A reduced MA toxicity in rat brains and primary VM cultures through an anti-apoptotic pathway. We found that either pre or co- administered AP4A with MA reduces the toxicity of MA in vivo or in vitro. Pretreatment with AP4A would have clinical significance in patients addicted to MA. Administration of AP4A may prophylactically reduce MA –mediated neurodegeneration in chronic MA abusers. It is also likely that AP4A may be clinically useful for immediate treatment of acute MA toxicity since co-treatment with AP4A can reduce MA toxicity. Further studies on AP4A mechanisms of action may provide the basis for novel therapeutic strategies for human neurodegenerative diseases.
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute on Drug Abuse, National Institutes of Health. The authors would like to thank Dr. Jean Lud Cadet for his comments and Ms. Kathleen Powers for her technical assistance.
Nonstandard abbreviations used
- AP4A
diadenosine tetraphosphate
- DIV
days in vitro
- DA
dopamine
- BSA
bovine serum albumin
- 6-OHDA
6-hydroxydopamine
- MA
methamphetamine
- PFA
paraformaldehyde
- SNpr
substantia nigra pars reticulata
- SNpc
substantia nigra pars compacta
- TUNEL
Terminal deoxynucleotidyl transferase (TdT)-mediated dNTP nick end labeling
- TH
tyrosine hydroxylase
- THir
tyrosine hydroxylase immunoreactivity
- VM
ventral mesencephalon
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali SF, Newport GD, Slikker W., Jr Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Ann N Y Acad Sci. 1996;801:187–98. doi: 10.1111/j.1749-6632.1996.tb17441.x. [DOI] [PubMed] [Google Scholar]
- Bochner BR, Lee PC, Wilson SW, Cutler CW, Ames BN. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984;37:225–32. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260:817–24. [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–80. [PubMed] [Google Scholar]
- Cadet JL, Ordonez SV, Ordonez JV. Methamphetamine induces apoptosis in immortalized neural cells: protection by the proto-oncogene, bcl-2. Synapse. 1997;25:176–84. doi: 10.1002/(SICI)1098-2396(199702)25:2<176::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J. 2003;17:1775–88. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Chou J, Harvey BK, Ebendal T, Hoffer BJ, Wang Y. Nigrostriatal alterations in bone morphogenetic protein receptor II dominant negative mice. Acta Neurochir Suppl. 2008a;101:93–8. doi: 10.1007/978-3-211-78205-7_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Luo Y, Kuo CC, Powers K, Shen H, Harvey BK, Hoffer BJ, Wang Y. Bone morphogenetic protein-7 reduces toxicity induced by high doses of methamphetamine in rodents. Neuroscience. 2008b;151:92–103. doi: 10.1016/j.neuroscience.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93:64–9. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL. Methamphetamine induces apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacology. 2002;42:837–45. doi: 10.1016/s0028-3908(02)00034-5. [DOI] [PubMed] [Google Scholar]
- Emanuelli T, Bonan CD, Sarkis JJ, Battastini AM. Catabolism of Ap4A and Ap5A by rat brain synaptosomes. Braz J Med Biol Res. 1998;31:1529–32. doi: 10.1590/s0100-879x1998001200003. [DOI] [PubMed] [Google Scholar]
- Emdadul HM, Asanuma M, Higashi Y, Miyazaki I, Tanaka K, Ogawa N. Apoptosis-inducing neurotoxicity of dopamine and its metabolites via reactive quinone generation in neuroblastoma cells. Biochim Biophys Acta. 2003;1619:39–52. doi: 10.1016/s0304-4165(02)00440-3. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238–51. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 2005;102:868–73. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev LL, Justesen J, Wolfson AD, Frolova LY. Diadenosine oligophosphates (Ap(n)A), a novel class of signalling molecules? FEBS Lett. 1998;427:157–63. doi: 10.1016/s0014-5793(98)00420-7. [DOI] [PubMed] [Google Scholar]
- Oaknin S, Rodriguez-Ferrer CR, Aguilar JS, Ramos A, Rotlly P. Receptor binding properties of di (1,N6-ethenoadenosine) 5′, 5′″-P1, P4-tetraphosphate and its modulatory effect on extracellular glutamate levels in rat striatum. Neurosci Lett. 2001;309:177–80. doi: 10.1016/s0304-3940(01)02067-5. [DOI] [PubMed] [Google Scholar]
- Pintor J, Porras A, Mora F, Miras-Portugal MT. Amphetamine-induced release of diadenosine polyphosphates--Ap4A and Ap5A--from caudate putamen of conscious rat. Neurosci Lett. 1993;150:13–6. doi: 10.1016/0304-3940(93)90096-4. [DOI] [PubMed] [Google Scholar]
- Pintor J, Porras A, Mora F, Miras-Portugal MT. Dopamine receptor blockade inhibits the amphetamine-induced release of diadenosine polyphosphates, diadenosine tetraphosphate and diadenosine pentaphosphate, from neostriatum of the conscious rat. J Neurochem. 1995;64:670–6. doi: 10.1046/j.1471-4159.1995.64020670.x. [DOI] [PubMed] [Google Scholar]
- Pintor J, Miras-Portugal MT. A novel receptor for diadenosine polyphosphates coupled to calcium increase in rat midbrain synaptosomes. Br J Pharmacol. 1995;115:895–902. doi: 10.1111/j.1476-5381.1995.tb15894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J, Carracedo G, Alonso MC, Bautista A, Peral A. Presence of diadenosine polyphosphates in human tears. Pflugers Arch. 2002;443:432–6. doi: 10.1007/s004240100696. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Misgeld U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J Neurosci. 1998;18:2009–16. doi: 10.1523/JNEUROSCI.18-06-02009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet P, Mele A, mmassari-Teule M. Involvement of glutamatergic and dopaminergic systems in the reactivity of mice to spatial and non-spatial change. Psychopharmacology (Berl) 1996;126:55–61. doi: 10.1007/BF02246411. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Bowyer JF. Methamphetamine exposure can produce neuronal degeneration in mouse hippocampal remnants. Brain Res. 1997;759:135–40. doi: 10.1016/s0006-8993(97)00173-x. [DOI] [PubMed] [Google Scholar]
- Stumm G, Schlegel J, Schafer T, Wurz C, Mennel HD, Krieg JC, Vedder H. Amphetamines induce apoptosis and regulation of bcl-x splice variants in neocortical neurons. FASEB J. 1999;13:1065–72. doi: 10.1096/fasebj.13.9.1065. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Abercrombie ED. Amphetamine-induced release of dendritic dopamine in substantia nigra pars reticulata: D1-mediated behavioral and electrophysiological effects. Synapse. 1996;23:280–91. doi: 10.1002/(SICI)1098-2396(199608)23:4<280::AID-SYN6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chang CF, Morales M, Chiang YH, Harvey BK, Su TP, Tsao LI, Chen S, Thiemermann C. Diadenosine tetraphosphate protects against injuries induced by ischemia and 6-hydroxydopamine in rat brain. J Neurosci. 2003;23:7958–65. doi: 10.1523/JNEUROSCI.23-21-07958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo N, Angulo JA. Methamphetamine-induced striatal apoptosis in the mouse brain: comparison of a binge to an acute bolus drug administration. Neurotoxicology. 2006;27:131–6. doi: 10.1016/j.neuro.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]