Abstract
Passive immunotherapy of cancer, i.e., transfer of T cells or antibodies, can lead to some objective clinical responses, thus demonstrating that the immune system can reject tumors. However, passive immunotherapy is not expected to yield memory T cells that might control tumor outgrowth. Active immunotherapy with dendritic cell (DCs) vaccines has the potential to induce tumor-specific effector and memory T cells. Clinical trials testing first generation DC vaccines pulsed with tumor antigens provided a proof-of-principle that therapeutic immunity can be elicited. Newer generation DC vaccines are build on the increased knowledge of the DC system including the existence of distinct DC subsets and their plasticity all leading to generation of distinct types of immunity. Rather than the quantity of IFN-γ secreting CD8+ T cells, we should aim at generating high quality high avidity poly-functional effector CD8+ T cells able to reject tumors and long-lived memory CD8+ T cells able to prevent relapse.
Keywords: dendritic cells, cancer, vaccines, priming
INTRODUCTION
Identification of T cell-defined tumor antigens in humans and development of technologies to produce monoclonal antibodies have facilitated the development of passive immunotherapy protocols. Antibodies, such as Rituxan (anti-CD20) or Herceptin (anti-her2/neu) are common practice in oncology. Adoptively transferred T cells can cause regression of established tumors in patients further demonstrating the role of the immune system in the control of malignancy. Yet, passive immunotherapy is not expected to yield long-lived memory T cells that might control tumor outgrowth. In contrast, active immunotherapy with vaccines has the potential to induce both tumor-specific effectors and memory T cells.1, 2
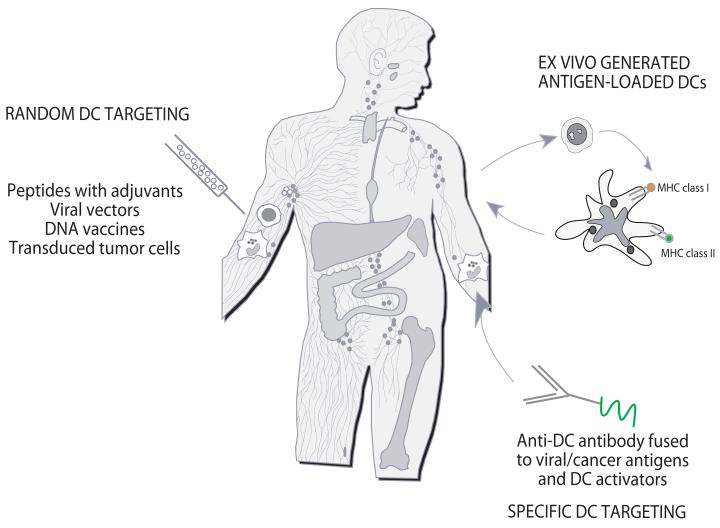
Vaccination relies on dendritic cells (DCs), the professional antigen presenting cells that induce and regulate immune responses. To allow resistance to infection and tolerance to self, DCs have evolved two cardinal features, i.e., functional plasticity and subsets.3 This diversity permits the adaptive immune system to mount functionally distinct types of responses. This is particularly important when considering DCs for vaccination. DCs can be randomly targeted in vivo with antigen and adjuvant, for example MHC class I peptides mixed with adjuvant (Figure 1). DCs can be generated ex-vivo from bone marrow progenitors or blood precursors and loaded with antigens for injection to patients. Finally, DCs can be specifically targeted in vivo with anti-DC antibodies decorated with antigens. A limitation of random targeting is that the capture of the vaccine antigen by an “undesired” DC subset might induce an unwanted type of immunity, for example a non-protective antibody response, or non-cytolytic CD8+ T cells rather than an effective CTL response. Ex-vivo generated DCs offer an opportunity to establish many of the parameters of DC vaccination including: i) the DC subset; ii) the antigen formulation, and iii) the activation signals. DCs targeting in vivo represents an attractive alternative. The proof-of-principle for this strategy comes from the work of Nussenzweig and Steinman who demonstrated in experimental models that minute amounts of antigen can be delivered to DCs via DEC-205 leading to generation of protective immunity against microbes as well as cancer.4
Figure 1. Three approaches to DC-based immune intervention in cancer.
1) Vaccines based on antigen with or without adjuvant that target DCs randomly. That might result in vaccine antigens being taken up by a “wrong” type of DCs in the periphery which might lead to “unwanted” type of immune response. Vaccine antigens could also flow to draining lymph nodes where they can be captured by resident DCs; 2) Vaccines based on ex-vivo generated tumor antigen-loaded DCs that are injected back into patients; and 3) specific in vivo DC targeting with anti-DC antibodies fused with antigens and with DC activators.
DENDRITIC CELLS IN VACCINATION AGAINST CANCER
First generation DC vaccines
Ex vivo-generated and antigen-laden DCs have now been used as vaccines to improve immunity in patients with cancer and HIV infection. These early studies have been discussed in details elsewhere.5 Hereunder, we briefly summarize our key conclusions from phase I/II clinical trials in patients with metastatic melanoma (Figure 2). In our trials, DCs were injected subcutaneously at three different sites to target as many lymph nodes as possible and thus increase the odds for eliciting antigen-specific immunity.
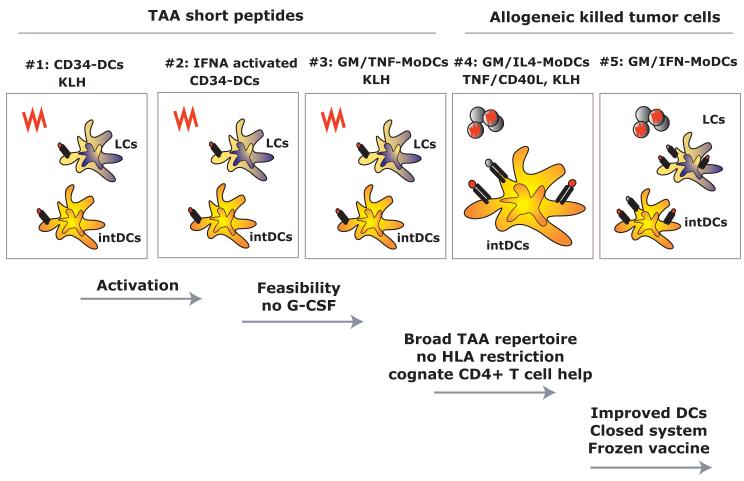
Figure 2. BIIR dendritic cell vaccine trials.
Illustrated are early trials conducted at BIIR. Early trials (#1-3) tested the immune and clinical efficacy of DC vaccines loaded with short peptides representing tumor associated antigens (TAA) and control antigens. These trials tested the immunogenicity of composite DC vaccines (containing Langerhans cells and interstitial DCs) (#1), activation of CD34+ HPCs-derived DCs (#2) and the activity of composite monocyte-derived DCs generated with GM-CSF and TNF (3#). Trial #4 tested the immune and clinical efficacy of monocyte-derived DCs loaded with killed allogeneic melanoma cells. Newer trials (#5 and beyond) are testing the immunogenicity of GM-CSF/IFN-DCs derived from monocytes.
1) Vaccination with melanoma peptide-loaded CD34-DCs can expand multiple antigen-specific T cells and improve their function
Our first vaccine was made of DCs generated by culturing CD34+ hematopoietic progenitor cells (CD34+ HPCs) with GM-CSF, TNF and FLT3 ligand.6 These CD34-DCs contain LCs. CD34-DCs were loaded with i) control antigens KLH and Influenza matrix (FluM1) peptide; and ii) multiple HLA-A*0201 binding 9-10AA peptides derived from MART-1/MelanA; gp100; tyrosinase and MAGE3. Initially, patients received four vaccinations over 6 weeks. In the second phase of the trial, patients received four additional boost vaccinations spread over four months or longer.
We concluded that antigen-laden CD34-DCs can promptly induce CD4+ and CD8+ T cell responses in vivo.6 Altogether, an enhanced immune response to multiple melanoma antigens (>2) measured in either direct or recall IFN-γ ELISPOT was observed in ten out of 16 immunologically responding patients. Importantly, in 9 out of 12 analyzed patients the expansion of cytolytic CD8+ T cell precursors (CTLp) specific for melanoma differentiation antigens was observed.7 These results permitted us to conclude that vaccination with DCs can increase the frequency and improve the function of melanoma antigen-specific CD8+ T cells. Furthermore, boosting vaccination with peptide-loaded CD34-DCs can expand long-lived tumor peptide-specific immunity.8
2) Vaccination with DCs loaded with killed allogeneic melanoma cells can elicit melanoma-specific immune responses
Loading DCs with exogenous peptides that bind MHC class I molecules can generate a limited set of high quality antigen-specific CD8+ T cells. Peptides are however limited to certain HLA types and to known tumor antigens. Loading DCs with killed tumor cells builds upon the unique capacity of DCs to present internalized antigens via MHC class I leading to cross-priming of naïve CD8+ T cells. A major advantage is that it is not limited to a selected haplotype such as HLA-A*0201. It also has the potential to allow presentation of tumor antigens on MHC class II molecules for tumor specific help. The second vaccine we tested was made of DCs generated by culturing enriched monocytes with GM-CSF and IL-4. DCs were loaded with whole tumor cells (killed Colo829 melanoma cells) to exploit their capacity for cross-priming.9 They were also loaded with KLH and activated with TNF and CD40 ligand. Patients received eight vaccinations on a monthly schedule.
We found type 1 CD8+ T cell immunity to MART-1 in three out of 13 analyzed patients.9 Two of these patients showed improved immunity in response to vaccination with DCs. Indeed, increased IFN-γ secretion and/or T cell proliferation in response to MART-1 derived peptides indicated in vivo cross-presentation of melanoma antigens by DC vaccine. In one patient, vaccination led to elicitation of IFN-γ producing CD8+ T cells specific to a MART-1 peptide to which no response could be detected at the onset. This suggests that in-vivo DC vaccines loaded with killed allogeneic melanoma cells are capable of cross-priming.
3) Relation between immune and clinical responses
Our studies and those of others indicate that the ability to mount immune responses to tumor antigens presented by DCs might be clinically important. Altogether, three groups of patients emerge:
Patients who do not mount immune response to melanoma antigens presented on DC vaccines. These patients are usually early clinical progressors. Their responses to control antigens such as KLH or viral peptides (Flu-M1 or CMV) are preserved in most cases indicating that they cannot mount tumor-specific responses.
Patients who show melanoma-antigen specific immunity but do not experience durable objective tumor regression: possibly the most common outcome of current DC vaccination protocols. Three mechanisms might be considered: a) the limited quality of immune responses induced with the first generation of DC vaccines; b) altered trafficking of induced T cells into the tumor lesions; and c) inhibition of effector T cell functions in the tumor microenvironment. Improved immunomonitoring in blood and at the tumor site using modern system biology approaches rather than the analysis of a single cytokine and/or frequency of tetramer positive cells will help resolve these questions.
Patients who mount immune responses and experience clinical benefit.
In our cross-priming trial, immune responses were associated in two patients with meaningful objective tumor regressions, i.e., durable CR (18 months) and a near CR (55+ months) in patients who failed other therapies while in stage IV (overall 10% objective response rate).9 Thus, vaccination with DCs can elicit therapeutic immunity and our challenge is to identify approaches that will enlarge the fraction of patients that can experience durable tumor regression.
The analysis of long-term outcomes in CD34-DCs trial revealed an association between the breadth of melanoma specific immunity and survival, i.e., patients who survived longer are those who showed immune response against more than 2 melanoma antigens presented on the DC vaccine.10 Three of these patients who were vaccinated between March 1999 and 2000 show no evidence of disease and are still alive in June 2009. These patients completed boosting vaccinations possibly arguing that immunologically responding patients should be vaccinated for a long time to maintain the pool of tumor antigen-specific T cells. Altogether, our retrospective analysis of overall survival in a cohort of 66 patients accrued between 1999 and 2003 shows 20% long-term survival. These data need now to be confirmed in prospective randomized trials testing survival as a pre-defined clinical endpoint.
Newer generation DC vaccines
Newer generation DC vaccines are build on the increased knowledge of the DC system including the existence of distinct DC subsets and their plasticity all leading to generation of distinct types of immunity. Rather than the quantity of IFN-γ secreting CD8+ T cells, we should aim at generating high quality high avidity poly-functional effector CD8+ T cells able to reject tumors and long-lived memory CD8+ T cells able to prevent relapse.
1) Plasticity of DCs and their precursors as key determinant of the type of immunity
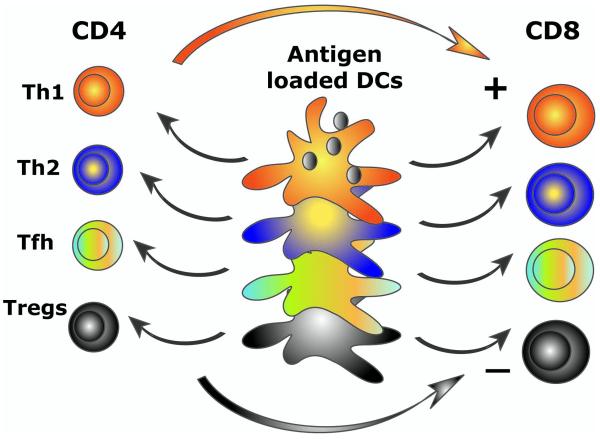
Several groups have now used IL4-DCs as vaccines following pioneering clinical studies in patients with metastatic melanoma by Nestle et al. 11 (using tumor-lysate-loaded DCs) and by Schuler and colleagues 12 (using melanoma-peptide-loaded DCs). However, the discoveries of past seven years point to alternatives to the classical way of generating DCs. These are based on two major concepts: 1) the plasticity of DC precursors; and 2) the plasticity of DCs DCs control lymphocyte priming and the type of induced T cell immunity (Figure 3). Distinct DC subsets are endowed with distinct functional properties as discussed by Banchereau et al in the same volume. Briefly, skin-derived and in vitro generated LCs and interstitial DCs differ in their capacity to activate lymphocytes. Interstitial DCs induce the differentiation of naïve B cells into immunoglobulin-secreting plasma cells and trigger differentiation of follicular helper T cells Tfh which promote antibody responses and isotype switch.13 LCs seem to be particularly efficient activators of cytotoxic CD8+ T cells.13
Figure 3. Distinct DC subsets generate distinct types of T cell immunity.
DC system has two cardinal features: 1) subsets; and 2) plasticity. This yields distinct types of immunity thereby allowing DCs to cope with protection against a variety of microbes and maintenance of tolerance to self. Understanding these two features is fundamental to develop vaccines that elicit the desired type of immune responses.
Different cytokines skew the in vitro differentiation of monocytes into different DCs. Thus, when activated (for example by GM-CSF) monocytes encounter IL-4 they will yield IL4-DCs.14 By contrast, after encounter with IFN-α/β, TSLP, TNF or IL-15, activated monocytes will differentiate into IFN-DCs, TSLP-DCs, TNF-DCs or IL15-DCs, respectively.15 This spectrum of DCs represents immunostimulatory DCs which generate different types of immune responses. For example, melanoma-peptide-pulsed IL15-DCs are much more efficient than IL4-DCs for the induction of antigen-specific CTL differentiation in vitro.16 Also, IFN-α-DCs generated in three-day cultures are efficient for the induction of specific immunity. Thus, the immunogenicity of these distinct DC vaccines needs to be tested in vivo in clinical studies. There also exists a whole repertoire of DCs that exhibit immunoregulatory functions, for example DCs generated by culturing monocytes in the presence of IL-10 are highly efficient in generation of anergic T cells and expansion of suppressor T cells.17 Thus, distinct DCs will induce distinct types of T cell immunity. The challenge is to link these distinct DC phenotypes in vitro with a specific type of immune response and immune pathology in vivo as exemplified by TNF and IFN-α in autoimmunity or by TSLP in allergic inflammation.18
DCs can receive maturation signals through several pathways including: i) microbes which act on DCs via Toll receptors (TLRs), C type lectins 19 and intracytoplasmic NOD-like receptors (NLRs) 20; ii) cells including T cells, NK cells, NKT cells and γ/δ T cells 21; iii) cell products such as CD40 ligand and proinflammatory cytokines including IL-1β, TNF, IL-6 and PGE2; and iv) products of dying cells, called damage-associated molecular pattern molecules (DAMPs).22 The type of DC maturation signals has a strong impact of their capacity to elicit T cell immunity.23 For example, when compared side-by-side in vitro GM-CSF/IL-4 DCs activated with a cocktail of IFN-α, polyI:C, IL-1β, TNF, and IFN-γ induce up to 40-fold higher numbers of melanoma-specific CTLs in a single round of in vitro sensitization than a “gold standard” DCs matured by a cocktail of macrophage cytokines including IL-1β/TNF/IL-6/prostaglandin E2 (PGE2).24 Furthermore, PGE2 can block the generation of bio-active IL12p70 by maturing DCs thus impacting the differentiation of type 1 helper T cells and promoting Th2 cells.25, 26 Thus, the conventional “gold standard” DC vaccines might not be optimal and need to be revisited.
2) Loading DC vaccines with antigen
We discussed above antigen preparations for loading DCs, for example peptides or killed tumor cells. An important issue to consider is also the nature of antigen used for immunization. Classical tumor antigens include: i) unique (mutated) antigens; and ii) shared self-antigens including cancer/testis antigens and tissue differentiation antigens. The choice between these types of antigens for vaccination could be viewed as choice between inducing immunity (mutated antigens) or breaking tolerance and inducing autoimmunity (self antigens). The potential of mutated antigens is opposed to i) relatively weak immunogenicity of shared antigens; and ii) the existence of T regs with specificity for shared antigens.27 An important shift in the selection of antigen targets is brought about by the identification of cancer stem cells. The importance of stem cell associated antigens in malignancy can be best illustrated by the presence of SOX-2-specific immunity in patients with monoclonal gammopathy.28 This immunity is lost in patients who developed multiple myeloma suggesting differential antigenic targets at pre-malignant and malignant stages.
3) Regulatory/suppressive pathways
A major obstacle to the success of cancer vaccines might be the presence of regulatory T cells (Tregs) and suppressive pathways established by tumors. Indeed, a large body of experimental evidence shows that Tregs suppress anti-tumor immunity and that their removal allows tumor eradication.29 Our studies in metastatic melanoma patients revealed the presence of circulating IL-10 producing CD4+ T cells specific for a broad range of melanoma antigens.30 These melanoma antigen-specific IL-10-secreting CD4+ T cells show suppressive activity in vitro though it appears to be IL-10 independent but contact dependent. It is conceivable that distinct DC subsets and/or distinct DC maturation stimuli will have different capacities to induce regulatory T cells. This aspect needs to be explored further. Naturally occurring CD4+CD25+ suppressor T cells may be controlled by pre-treatment of patients with drugs that can eliminate and/or control these cells. An example is combination of vaccination with low dose Cyclophosphamide which has been shown to impact suppressive cells in the mouse.31 The efficacy of Cytoxan to control Tregs in humans remains however to be established and several trials testing this drug in combination with vaccines are currently ongoing.
ASSESSING VACCINE EFFICACY
Assessing the quality rather than the quantity of immune responses
Vaccine-specific T cell immunity has been classically measured by the quantity of tumor-antigen specific CD8+ T cells using IFN-γ ELISPOT and tetramers. However, it becomes apparent that a single cytokine assay cannot serve as a measure of the quality of the induced immune responses, hence vaccine efficacy. This is likely one of the reasons why we still cannot define the parameters of protective immunity in cancer. Thus the important parameter of vaccine efficacy is the quality and not the quantity. Our understanding as to what makes an effective CTL stems from studies including i) adoptive T cell transfer in patients with cancer32, 33; ii) vaccination studies with careful immunomonitoring; and iii) studies in HIV infected patients.34 The consensus is that CD8+ T cells need to fulfill a set of criteria: 1) Polyfunctional at the level of cytokine secretion, as only those capable of secreting several cytokines including IFN-γ, TNF and IL-2 can control HIV viral load;35 2) High avidity;36 3) Able to kill authentic tumor targets, which present natural levels of TCR ligands and low levels of costimulatory molecules, rather than peptide-pulsed T2 cells;37 4) The capacity to reject tumors is linked with the expression of granzyme B and perforin;38 5) Long lived memory CD8+ T cells. Developing validated assays and criteria permitting us to compare different studies is not a trivial effort. Hence, The International Society for the Biological Therapy of Cancer (iSBTc) has initiated in collaboration with the United States Food and Drug Administration (FDA) a programmatic look at innovative avenues and validation studies for the identification of relevant parameters that can be used to assess the natural course of host/tumor interactions or their response to immune manipulation.39
Global and unbiased approaches to analyze immune responses are needed to determine immune correlates of clinical efficacy. With this in mind, we developed two approaches: 1) blood transcriptional profiling; and 2) EPIMAX, which allows a global measurement of antigen-specific immune repertoire.9, 30 Blood transcriptional signatures have already been identified for a wide spectrum of diseases including autoimmune diseases such as lupus.40 Using microarrays in patient-based studies also led to the identification of IL1 as a novel therapeutic target for the treatment of systemic onset juvenile arthritis, ultimately leading to the development of a new treatment modality and set of diagnostic markers.41 More recently we described the development of a modular transcriptional framework as a basis for the identification of blood biomarker signatures.42 Pulendran and Sekaly’s laboratories have recently shown that administration of the Yellow Fever Vaccine to healthy individuals induces a remarkable transcriptome signature in the blood which peaks seven days after vaccine administration.43, 44 Interestingly, individuals who show a predominance of Humoral Immunity show a signature based around the B cell growth factor TNFRS17 (BAFF/Blys). In contrast, patients who show a preferential Cellular Immunity response, show a signature centered around the complement protein C1qB and eukaryotic translation initiation factor2 alpha kinase 4.
EPIMAX measures simultaneously cell proliferation and secretion of multiple cytokines that distinguish Type 1, Type 2 cytokines and IL-10 secretion using the Multiplex Cytokine Analysis. This technology permitted us to measure directly in the blood melanoma antigen-specific IL-10 secreting T cells.30 Furthermore, this technology might permit us to measure the baseline status of tumor-specific immunity in patients and therefore identify patients with pre-existing immunity that might benefit the most from vaccination therapy. Thus, transcriptional profiling and EPIMAX in combination with polychromatic flow cytometry will permit us to gain a better insight. The challenge will be to develop bioinformatics strategies to exploit the data.
Assessing Clinical efficacy
The definition of clinical endpoints, and hence the measures that are used to assess vaccine efficacy, need to be revisited. Cancer is a chronic disease; therefore, prolonged survival and good quality of life might be considered a therapeutic success and of benefit to the patient. Thus, while critically assessing different therapies, we should be careful not to pre-maturely dismiss therapies that do not lead to a high rate of objective tumor regression. Furthermore, it might be considered unrealistic to expect even the most efficient immune responses to eliminate the total tumor burden in a patient with advanced cancer.
CONCLUDING REMARKS
DCs are the critical decision-making cells in the immune response. DCs are an attractive target for therapeutic manipulation of the immune system to enhance otherwise insufficient immune responses to tumor antigens. However, the complexity of the DC system requires their rational manipulation to achieve protective or therapeutic immunity. Thus, further research is needed to analyze the immune responses induced in patients by distinct ex vivo generated DC subsets activated via different pathways. DC-based vaccination will become an essential component in cancer management. The considerable progresses made in the knowledge of DC biology as well as effector/regulatory T cell biology clearly open the avenues for development of considerably improved clinical protocols.
The ultimate ex vivo-generated therapeutic DC vaccine will be heterogeneous and composed of several subsets, each of which will target a specific immune effector. These ex vivo strategies should help to identify the parameters for DC targeting in vivo, which represents the next step in the development of DC-based vaccination, most particularly for preventive vaccination. These novel vaccines will be based on in vivo targeting of DCs with fusion proteins containing anti-DCs antibodies, antigens from tumor stem/propagating cells and DC activators (Figure 4).
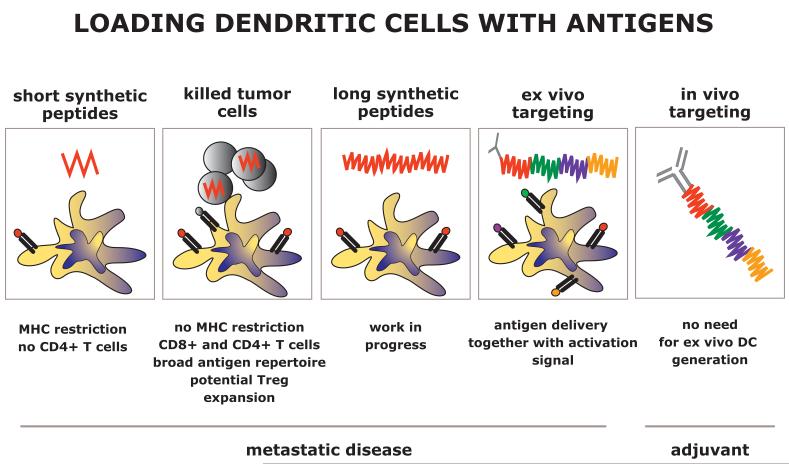
Figure 4. Loading DCs with tumor antigens.
In our initial trials, DCs were loaded with short synthetic peptides, the major limitations of which are MHC restriction and the lack of cognate CD4+ T cell epitopes. Next step was to test loading DCs with killed tumor cells. These are able to generate a broad repertoire of CD4+ and CD8+ T cells. Long synthetic peptides might overcome MHC restriction and allow us to better control T cell repertoire. These studies will lay the ground for selection of long peptides that can be fused with anti-DC antibodies for ex vivo and in vivo DC targeting.
Acknowledgments
This manuscript is dedicated to all of our patients and volunteers who have participated in our studies and clinical trials. We thank former and current members of the Institute for their contributions to our progresses; Susan Burkeholder, Jennifer Finholt, Nicolas Taquet, and Susan Hicks for excellent work and commitment to DC vaccination trials; Dr. Lee Roberts for help; Dr. Carson Harrod for proofreading the manuscript; Cindy Samuelsen for continuous help; and Dr. Michael Ramsay for continuous support. Supported by the NIH (P01 CA084514, R01 CA089440 and CA078846), the Dana Foundation, the Susan Komen Foundation, the Baylor Health Care System and the Baylor Health Care System Foundation. AKP holds the Michael A. Ramsay Chair for Cancer Immunology Research. JB holds the Caruth Chair for Transplant Immunology Research.
References
- 1.Finn O. Cancer Immunology. N Engl J Med. 2008;358:25. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 2.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373:673–683. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 4.Bonifaz LC, et al. In Vivo Targeting of Antigens to Maturing Dendritic Cells via the DEC-205 Receptor Improves T Cell Vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 7.Paczesny S, et al. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J Exp Med. 2004;199:1503–1511. doi: 10.1084/jem.20032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palucka AK, et al. Boosting Vaccinations with Peptide-Pulsed CD34+ Progenitor-Derived Dendritic Cells Can Expand Long-Lived Melanoma Peptide-Specific CD8+ T Cells in Patients with Metastatic Melanoma. J Immunother. 2005;28:158–168. doi: 10.1097/01.cji.0000154249.74383.17. [DOI] [PubMed] [Google Scholar]
- 9.Palucka AK, et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 10.Fay JW, et al. Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34(+) progenitor-derived dendritic cells. Cancer Immunol Immunother. 2005:1–10. doi: 10.1007/s00262-005-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nestle FO, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 12.Thurner B, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romani N, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 16.Dubsky P, et al. IL-15-induced human DC efficiently prime melanoma-specific naive CD8(+) T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 17.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 18.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 19.Cambi A, Figdor CG. Levels of complexity in pathogen recognition by C-type lectins. Curr Opin Immunol. 2005;17:345–351. doi: 10.1016/j.coi.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 21.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 23.Koski GK, Cohen PA, Roses RE, Xu S, Czerniecki BJ. Reengineering dendritic cell-based anti-cancer vaccines. Immunol Rev. 2008;222:256–276. doi: 10.1111/j.1600-065X.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 24.Mailliard RB, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 25.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 26.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–3469. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 27.Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J. Immunol. 2007;178:1975–1979. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- 28.Spisek R, et al. Frequent and specific immunity to the embryonal stem cell associated antigen SOX2 in patients with monoclonal gammopathy. J. Exp. Med. 2007;204:831–840. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steitz J, Bruck J, Lenz J, Knop J, Tuting T. Depletion of CD25(+) CD4(+) T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon alpha-induced, CD8(+) T-cell-dependent immune defense of B16 melanoma. Cancer Res. 2001;61:8643–8646. [PubMed] [Google Scholar]
- 30.Vence L, et al. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yee C, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma. Direct evidence of t cell-mediated vitiligo. J. Exp. Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nat Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 35.Precopio ML, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh S, et al. Selective induction of high avidity CTL by altering the balance of signals from APC. J Immunol. 2003;170:2523–2530. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 37.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 38.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 39.Tahara H, et al. Emerging concepts in biomarker discovery; The US-Japan workshop on immunological molecular markers in oncology. J Transl Med. 2009;7:45. doi: 10.1186/1479-5876-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allantaz F, et al. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. J Exp Med. 2007;204:2131–2144. doi: 10.1084/jem.20070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaussabel D, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29:150–164. doi: 10.1016/j.immuni.2008.05.012. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaucher D, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Querec TD, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]