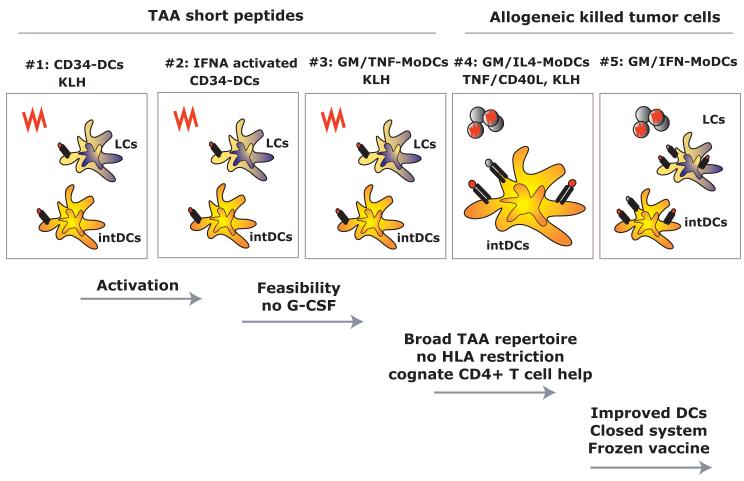
Figure 2. BIIR dendritic cell vaccine trials.
Illustrated are early trials conducted at BIIR. Early trials (#1-3) tested the immune and clinical efficacy of DC vaccines loaded with short peptides representing tumor associated antigens (TAA) and control antigens. These trials tested the immunogenicity of composite DC vaccines (containing Langerhans cells and interstitial DCs) (#1), activation of CD34+ HPCs-derived DCs (#2) and the activity of composite monocyte-derived DCs generated with GM-CSF and TNF (3#). Trial #4 tested the immune and clinical efficacy of monocyte-derived DCs loaded with killed allogeneic melanoma cells. Newer trials (#5 and beyond) are testing the immunogenicity of GM-CSF/IFN-DCs derived from monocytes.