Abstract
The intra vitam diagnosis of prion disease is challenging and a definite diagnosis still requires neuropathological examination in non-familial cases. Magnetic resonance imaging has gained increasing importance in the diagnosis of prion disease. The aim of this study was to compare the usefulness of different magnetic resonance imaging sequences and proton magnetic resonance spectroscopy in the differential diagnosis of patients with rapidly progressive neurological signs compatible with the clinical diagnosis of sporadic prion disease. Twenty-nine consecutive patients with an initial diagnosis of possible or probable sporadic prion disease, on the basis of clinical and electroencephalography features, were recruited. The magnetic resonance protocol included axial fluid-attenuated inversion recovery-T2- and diffusion-weighted images, and proton magnetic resonance spectroscopy of the thalamus, striatum, cerebellum and occipital cortex. Based on the clinical follow-up, genetic studies and neuropathology, the final diagnosis was of prion disease in 14 patients out of 29. The percentage of correctly diagnosed cases was 86% for diffusion-weighted imaging (hyperintensity in the striatum/cerebral cortex), 86% for thalamic N-acetyl-aspartate to creatine ratio (cutoff ≤1.21), 90% for thalamic N-acetyl-aspartate to myo-inositol (mI) ratio (cutoff ≤1.05) and 86% for cerebral spinal fluid 14-3-3 protein. All the prion disease patients had N-acetyl-aspartate to creatine ratios ≤1.21 (100% sensitivity and 100% negative predictive value) and all the non-prion patients had N-acetyl-aspartate to myo-inositol ratios >1.05 (100% specificity and 100% positive predictive value). Univariate logistic regression analysis showed that the combination of thalamic N-acetyl-aspartate to creatine ratio and diffusion-weighted imaging correctly classified 93% of the patients. The combination of thalamic proton magnetic resonance spectroscopy (10 min acquisition duration) and brain diffusion-weighted imaging (2 min acquisition duration) may increase the diagnostic accuracy of the magnetic resonance scan. Both sequences should be routinely included in the clinical work-up of patients with suspected prion disease.
Keywords: prion diseases, magnetic resonance, diffusion-weighted imaging, proton MR spectroscopy
Introduction
Prion diseases are rare, fatal, neurodegenerative disorders mainly affecting the central nervous system, which can be familial, sporadic or acquired by infection (Gambetti et al., 2003). The intra vitam diagnosis is challenging as there is currently no non-invasive in vivo diagnostic test and a definite diagnosis still requires neuropathological examination in non-familial cases. The initial diagnostic suspicion of prion disease is clinical and usually raised in the presence of rapidly progressive neurological signs not associated with focal neuroradiological abnormalities. Two paraclinical tests are currently used to increase the clinical diagnostic sensitivity and specificity: electroencephalography (EEG) recording and testing the cerebrospinal fluid (CSF) for 14-3-3 protein (Zerr et al., 2000). Patients with Creutzfeldt–Jakob disease (CJD), the most common human prion disease, may show periodic sharp and slow wave complexes in the EEG and, at a higher rate, increased CSF levels of the 14-3-3 protein. Sensitivity and specificity of periodic sharp and slow wave complexes (66 and 74%, respectively) are lower compared with the 14-3-3 analysis, which showed an overall sensitivity of 94% and specificity of 84% (Zerr et al., 2000; Steinhoff et al., 2004). On the other hand, when the 14-3-3 test was used on unselected patients with dementia, half of them rapidly progressive (disease duration ≤12 months), a rate of 12% false positive cases was revealed (Burkhard et al., 2001). Furthermore, fatal insomnia, a rare atypical form of sporadic prion disease, lacks increased CSF levels of the 14-3-3 protein in nearly all cases (Krasnianski et al., 2008).
Magnetic resonance imaging (MRI) has gained increasing importance in the diagnosis of prion diseases (Collie et al., 2001; Tschampa et al., 2007). A symmetric hyperintense signal in the caudate nucleus and putamen is regarded as typical, particularly on fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted images (DWI) (Bahn and Parchi 1999; Schröter et al., 2000; Shiga et al., 2004). In the largest MRI study available, 193 consecutive cases of suspected sporadic CJD were scanned and the diagnostic sensitivity ranged from 58% to 71% and specificity from 82% to 89%, depending on the observer (Tschampa et al., 2005).
Proton magnetic resonance spectroscopy (1H-MRS) allows for the non-invasive and spatially resolved measurement of several brain compounds including N-acetyl-aspartate (NAA), a neuronal marker (Kantarci et al., 2008), and myo-inositol (mI) a glial marker (Brand et al., 1993). The use of 1H-MRS in the investigation of sporadic CJD patients has been extremely limited. The study of individual cases has revealed reduced NAA in different brain areas including basal ganglia/thalamus (Bruhn et al., 1991; Pandya et al., 2003; Lim et al., 2004) and cortex (Bruhn et al., 1991; Graham et al., 1993; Lim et al., 2004). When short echo times were used, myo-inositol was quantified and found to be markedly increased (Bruhn et al., 1991). Similar changes have been reported in the basal ganglia/thalamus of patients with familial CJD (Waldman et al., 2006; Haik et al., 2008) and variant CJD (Cordery et al., 2006).
In the present study, we compared the usefulness of magnetic resonance techniques such as FLAIR-T2, DWI and 1H-MRS in the differential diagnosis of patients with rapidly progressive neurological signs compatible with the clinical diagnosis of sporadic prion disease (Zerr et al., 2000). Given the phenotypic and pathological heterogeneity of sporadic prion diseases, mainly related to codon 129 polymorphism and prion protein type, the neurochemical profile was obtained by 1H-MRS in four different brain areas, i.e. thalamus, striatum, cerebellum and occipital cortex, known to be most commonly affected in the different forms of sporadic prion disease (Parchi et al., 1999).
Methods
Study design
Patients were enrolled on the basis of clinical and electroencephalographic features (Steinhoff et al., 2004) (Fig. 1, Table 1). The clinical criteria of inclusion were based on modified WHO 1998 criteria (Zeidler et al., 1998) allowing for a single cognitive deficit, rapidly progressive cerebellar dysfunction in the absence of cognitive deficits and illness duration up to 36 months; features which have been shown to recur consistently among some sporadic human prion disease subtypes (Parchi et al., 1999). The patients who exhibited a rapidly progressive dementia, a single cognitive deficit or a rapidly progressive cerebellar dysfunction and two of the following: myoclonus, visual and cerebellar symptoms or both, pyramidal and extrapyramidal signs or both, or akinetic mutism were classified as probable prion disease in the presence of periodic sharp and slow wave complexes on EEG, and as possible prion disease if EEG was negative for periodic sharp and slow wave complexes. Results of 14-3-3 determination in CSF were not used for the initial diagnosis.
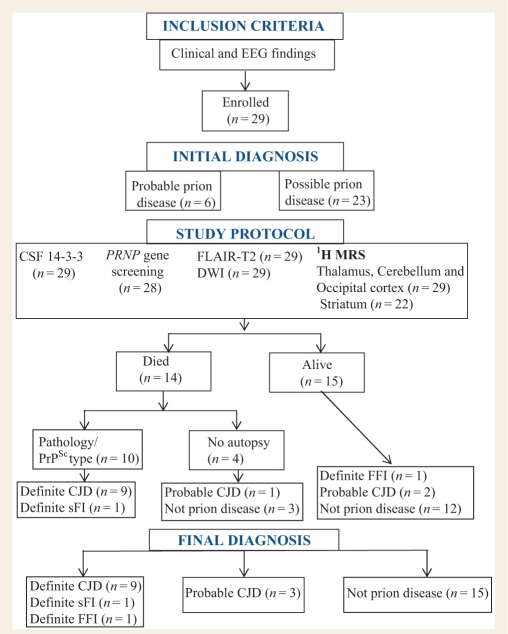
Figure 1.
Summary of subject enrolment and study design. sFI = sporadic fatal insomnia; FFI = familial fatal insomnia.
Table 1.
Clinical EEG, CSF and neuroradiological data of patients with definite/probable prion disease or without prion disease
| Case (n) | Age at onset/ sex | Duration of disease at MRI (months) | Dementia | C/V | P/EP | M | AM | EEG PSWC | Initial clinical and EEG diagnosis | CSF 14-3-3 | MRI | Final diagnosis | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with prion disease | |||||||||||||
| 1 | 72/M | 7 | Memory deficit | C | EP | + | + | − | Possible prion disease | + | + | Definite sCJD VV2 | Dead 6 m after onset/2 m after scan |
| 2 | 43/F | 11 | + | C | − | + | − | − | Possible prion disease | − | − | Definite sFI MM2 | Dead 23 m after onset/12 m after scan |
| 3 | 61/F | 3 | + | V | − | + | − | + | Probable prion disease | + | + | Definite fCJD V210I-129MM MM1 | Dead 4 m after onset/1 m after scan |
| 4 | 65/F | 3.5 | + | C | P | − | − | − | Possible prion disease | + | + | Definite sCJD VV2 | Dead 8 m after onset/4.5 m after scan |
| 5 | 40/M | 5 | Memory deficit | C/V | EP | + | + | − | Possible prion disease | + | + | Probable sCJD VV | Dead 7 m after onset/2 m after scan; autopsy not done |
| 6 | 61/F | 2 | + | C/V | EP | + | − | + | Probable prion disease | + | + | Definite sCJD MM1 | Dead 4 m after onset/2 m after scan |
| 7 | 49/F | 4.5 | − | C | − | + | − | − | Possible prion disease | + | + | Definite fCJD E200K-129MV MV2 | Dead 10 m after onset/5.5 m after scan |
| 8 | 74/F | 11 | + | V | P/EP | + | + | − | Possible prion disease | + | + | Definite sCJD MV2 | Dead 15 m after onset/4 m after scan |
| 9 | 65/F | 26 | + | − | P/EP | + | − | − | Possible prion disease | + | + | Definite sCJD MV2 | Dead 36 m after onset/10 m after scan |
| 10 | 78/M | 3 | Memory deficit | C | EP | + | + | − | Possible prion disease | + | + | Definite sCJD VV2 | Dead 7 m after onset/4 m after scan |
| 11 | 67/F | 5 | + | C/V | P | + | + | + | Probable prion disease | + | + | Probable sCJD MM | Alive; progressive worsening follow-up >1 years |
| 12 | 34/M | 16 | + | − | EP | + | − | − | Possible prion disease | + | + | Probable sCJD MV | Alive; progressive worsening follow-up >1 years |
| 13 | 69/M | 3 | + | V | − | + | − | + | Probable prion disease | + | + | Definite sCJD MM1 | Dead 4 m after onset/1 m after scan |
| 14 | 45/M | 11 | − | V/C | P | + | − | − | Possible prion disease | − | − | Definite FFI D178N-129MV | Alive; progressive worsening follow-up >1 years |
| Patients without prion disease | |||||||||||||
| 15 | 54/F | 9 | + | − | P | + | − | − | Possible prion disease | − | − | Possible Alzheimer disease | Alive; follow-up >2 years |
| 16 | 68/F | 3 | + | − | EP | + | − | + | Probable prion disease | − | − | Probable dementia with Lewy bodies | Alive; follow-up >2 years |
| 17 | 62/F | 24 | + | − | EP | + | − | − | Possible prion disease | − | − | Probable cortico-basal degeneration | Alive; follow-up >3 years |
| 18 | 56/M | 1 | + | − | EP | − | + | − | Possible prion disease | − | + | Possible autoimmune encephalitis | Asymptomatic after corticosteroid therapy; follow-up >2 years |
| 19 | 74/M | 4 | + | C | − | + | − | − | Possible prion disease | − | − | Possible autoimmune encephalitis | Dead 6 m from onset; autopsy not done |
| 20 | 63/M | 10 | + | − | P/EP | + | − | − | Possible prion disease | − | − | Paraneoplastic encephalitis | Dead 16 m from onset; autopsy not done |
| 21 | 64/M | 3 | + | C | − | + | − | Possible prion disease | − | − | Probable autoimmune encephalitis | Improvement after corticosteroid therapy; follow-up >2 years | |
| 22 | 66/M | 3 | + | − | P/EP | + | − | − | Possible prion disease | − | − | Probable autoimmune encephalitis | Improvement after corticosteroid therapy; follow-up >2 years |
| 23 | 81/F | 36 | Memory deficit | − | EP | + | − | − | Possible prion disease | − | − | Probable cortico-basal degeneration | Alive; follow-up > 2 years |
| 24 | 69/F | 12 | + | − | EP | + | − | − | Possible prion disease | − | − | Probable cortico-basal degeneration | Alive; follow-up > 2 years |
| 25 | 77/M | 6 | Progressive aphasia | − | P | + | − | − | Possible prion disease | − | − | Probable fronto-temporal dementia/ALS | Dead 7 m from onset; autopsy not done |
| 26 | 55/M | 24 | Memory deficit | + | P/EP | − | − | − | Possible prion disease | − | Atypic parkinsonism | Alive; follow-up > 2 years | |
| 27 | 64/M | 24 | + | − | P | + | − | − | Possible prion disease | − | − | f-Cerebral amyloidosis | Alive; follow-up > 2 years |
| 28 | 34/F | 5 | + | − | P | + | − | + | Probable prion disease | + | + | Unknown; clinical improvement | Alive; follow-up > 1 years |
| 29 | 63/M | 2 | + | C | − | + | − | − | Possible prion disease | + | − | Unknown; clinical improvement | Alive; follow-up > 0.5 years |
D = dementia; C/V = cerebellar/visual signs; P/EP = pyramidal/extrapyramidal signs; M = myoclonus; AM = akinetic mutism; EEG = the presence of periodic sharp and slow wave complexes is indicated with plus symbol; CSF = the increase in the content of 14-3-3 protein is indicated with plus symbol; MRI = basal ganglia and/or cortical hyperintensity on FLAIR and/or DWI is indicated with plus symbol; ALS = amyotrophic lateral sclerosis; sCJD = sporadic CJD; fCJD = familial CJD linked to PRNP mutation; PSWC = periodic sharp and slow wave complexes; sFI = sporadic fatal insomnia; FFI = familial fatal insomnia; m = month.
Subjects
Twenty-nine consecutive patients were recruited from June 2003 to December 2007 through the Department of Clinical Neurosciences of the University of Bologna and its related Neurology Services. Ten healthy volunteers (five males and five females) with ages ranging from 34 to 82 years (62 ± 12 years, mean ± SD) were also studied. All magnetic resonance scans were performed at the ‘MR Spectroscopy Unit’ of the Policlinico S. Orsola-Malpighi University Hospital in Bologna. For all recruited patients, CSF samples were analysed for the presence of 14-3-3 protein, and a genetic study of the human prion protein gene was carried out. A post-mortem examination was performed in 10 patients.
The study was approved by the Policlinico S. Orsola-Malpighi Hospital Ethics Committee, and written informed consent was obtained from each participant or relative, in accordance with the Declaration of Helsinki.
Magnetic resonance imaging and spectroscopy
Magnetic resonance studies were performed using a 1.5 T GE Signa Horizon LX system equipped with a birdcage head radio-frequency coil for signal reception and an EchoSpeed gradient system providing a maximum gradient strength of 22 mT/m and maximum slew rate of 120 mT/m/ms.
In all recruited patients, axial T1-weighted spin-echo (repetition time, TR = 500 ms; echo time, TE = 10 ms) and FLAIR (TR = 8000 ms, inversion time, TI = 2000 ms, TE = 93.5 ms) images were acquired with 5 mm thick slices and 1 mm inter-slice gap. DWI with the same slice spacing were obtained using a single-shot spin-echo planar imaging technique (α = 90°, TR = 10 s, TE = 100 ms). Gradient strengths were chosen corresponding to a b-factor value of 900 s/mm2.
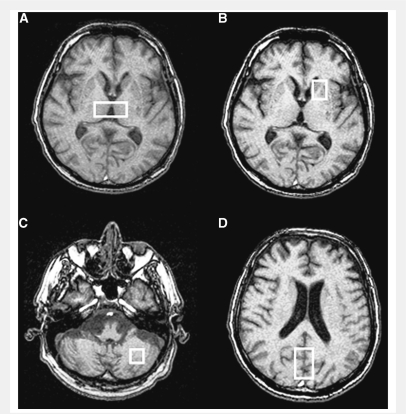
In order to carefully localize 1H-MRS regions of interest, contiguous 3 mm thick images were also acquired, using axial fast gradient-echo (TR = 250 ms; TE = 2.5 ms). Given the clinical and pathological heterogeneity of CJD, regions of interests were selected in brain areas known to be most frequently involved in the different forms of CJD (Parchi et al., 1999). Proton magnetic resonance spectra were acquired using the point resolved spectroscopy single voxel localization sequence (TE = 35 ms; TR = 4000 ms) with chemical shift selective imaging sequence water suppression. Four voxels were selected (Fig. 2), to include the bilateral dorso-medial thalamic nuclei (volume 4.0–5.0 cm3; 128 acquisitions), the left striatum (caudate head and anterior left putamen, volume 2.7–5.0 cm3; 128 acquisitions), mid-brain occipital cortex (volume 17.8–18.1 cm3; 32 acquisitions) and left cerebellum (volume 6.0–7.2 cm3; 64 acquisitions). Peak integrals for NAA, creatine-phosphocreatine (Cr), choline-containing compounds (Cho) and myo-inositol were calculated using the operator-independent fitting program LCModel with standard basis sets (Provencher, 1993). Peak integral values were expressed relative to Cr and, for NAA, to myo-inositol. The exclusion criterion for metabolite evaluation was an LCModel estimated fitting error >20%, this being a reliable indicator of poor quality spectra.
Figure 2.
Proton magnetic resonance spectra localization: (A) bilateral dorso-medial thalamus, (B) left striatum (caudate head and anterior putamen), (C) left cerebellum and (D) mid brain occipital cortex.
Western blot analysis of 14-3-3 protein
The 14-3-3 protein analysis was performed according to Castellani et al. (2004) with minor modifications. Briefly, 20 μl of CSF was added to 20 μl of 2× sample buffer and boiled for 10 min. Following sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) on 12% Tris–glycine Mini gels (Bio-Rad), protein was transferred to Immobilon P transfer membrane (Millipore). Membranes were then incubated with polyclonal anti-rabbit antibody to the β-isoform of 14-3-3 protein (Santa Cruz), followed by secondary antibody (donkey anti-rabbit IgG conjugated with horse radish peroxidase, Amersham), and developed using the ECL plus detection system (Amersham).
Screening of the prion protein gene, PRNP
The entire PRNP open reading frame was analysed by PRNP sequencing to determine the codon 129 genotype and the possible presence of mutations according to Parchi et al. (1999).
Neuropathologic post-mortem studies
The diagnoses of prion disease, including the specific subtype according to Parchi et al. (1999), were definitely established in the autopsied subjects by western blot analyses of PrPSc type from brain samples and by neuropathologic examination.
Evaluation of diagnostic tests
EEG was considered positive in the presence of periodic sharp and slow wave complexes (Steinhoff et al., 2004). The CSF 14-3-3 immunoassays were performed using western blot, with conformity of testing methods and interpretation of results confirmed by a blinded sample exchange programme. All samples were run in duplicate along with the following controls: positive (detectable 14-3-3 protein from confirmed CJD subjects), negative (undetectable 14-3-3 protein from confirmed non-CJD subjects) and ambiguous (from non-CJD subjects who had trace levels of 14-3-3 protein). The 14-3-3 test, evaluated by one rater (PP) with >5 years experience, was considered positive only when the 14-3-3 immunoreactivity was clearly stronger than that of the ambiguous sample. MRI brain scans were reviewed by one rater (RL), with a neuroradiological experience of >10 years, blind to the condition of the patients. The FLAIR and DWI were scored separately, for the presence or absence of bilateral increased signal intensity (SI) in the striatum, thalamus and cerebral cortex. High signal in the striatum and/or in the cerebral cortex were considered a positive finding (Collie et al., 2001; Tschampa et al., 2007).
Statistical analysis
Statistical analysis was performed with Statistical Package for Social Science Software (SPSS, version 13.0, Chicago, IL) for Windows.
One-way analysis of variance (ANOVA) was performed for comparison of 1H-MRS metabolite ratios and concentrations between the subject groups. The statistical comparison of spectroscopic values was performed using parametric tests, since a Kolmogorov–Smirnov analysis showed that all the variables were normally distributed (data not shown). The threshold of significance was set at P < 0.05. When it was determined that differences existed among the groups, Student's t-tests were performed to determine which mean values differed, using the Bonferroni correction for multiple comparisons. For each numerical MR spectroscopic variable, the optimal cutoff values, that gave the best balance between sensitivity and specificity, were determined by receiver operating characteristic curve analysis (Armitage et al., 2002). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and the number of cases correctly identified were calculated to determine the diagnostic capability of 1H-MRS variables, DWI and CSF 14-3-3 protein. Univariate logistic regression analysis, employing a backward stepwise inclusion method, was developed using a P-value of 0.05 to discriminate which variables affected the outcome. The regression coefficients and standard errors (SE) of the independent variables were calculated. Those coefficients not significantly different from zero were removed from the regression model. Finally, odds ratios (OR) and confidence intervals (CI) of variables included in the models were calculated, and a classification table was used to evaluate the predictive accuracy of the model.
Results
Clinical, electroencephalography and cerebrospinal fluid findings
Among the 29 patients recruited, on the basis of clinical and EEG features, 6 had an initial diagnosis of probable and 23 of possible prion disease.
A definite diagnosis of either CJD or fatal insomnia was made in 10 patients (Table 1). Among these patients, nine had a final diagnosis of definite CJD (three VV2, three MV2 and three MM1 patients) (Zerr et al., 2000) and one of sporadic fatal insomnia (MM2). Case #3 (MM1) and Case #7 (MV2) turned out to be genetic CJD cases, despite the lack of family history and apparent sporadic occurrence at clinical examination. Case #14 carried the D178N mutation and therefore was diagnosed as definite familial fatal insomnia even without post-mortem examination. In three patients (two still alive, Cases #11 and #12, and one dead without post-mortem examination, Case #5), all positive for 14-3-3 protein, the final diagnosis was, according to Zerr et al. (2000), probable CJD. As detailed in Table 1, the clinical follow-up excluded the diagnosis of prion disease in the remaining 15 patients who, in most cases, were affected by other neurodegenerative or inflammatory disorders. Two patients with probable (Case #28) and possible (Case #29) prion disease had a much shorter follow-up, but the diagnosis of prion disease could be excluded on the basis of a substantial clinical improvement from onset. All the non-prion patients, except Case #24 who was not tested, were negative for PRNP mutations.
EEG was positive for periodic sharp and slow wave complexes in 4/14 patients with prion disease and 2/15 patients without prion disease.
The 14-3-3 protein test in CSF was judged positive in 12/14 prion patients and in 2/15 non-prion patients. Among prion-affected subjects, the 14-3-3 protein test was negative in the two patients affected by the sporadic (Case #2) or familial (Case #14) subtype of fatal insomnia. Among non-prion patients, Cases #28 and #29 were false positive.
Magnetic resonance imaging and spectroscopy
The comparison of FLAIR-T2 and DWI SI changes in the striatum, thalamus and cerebral cortex for the 29 patients is reported in Table 2, and some example images demonstrating characteristic features are shown in Fig. 3. DWI clearly demonstrated a much higher sensitivity than FLAIR-T2 images in detecting SI increase in the thalamus and cerebral cortex. Among prion patients, DWI showed increased SI in the striatum of 12 cases, in the thalamus of 6 cases and in the cerebral cortex of 8 cases. Subjects #2 and #14, with sporadic and familial fatal insomnia, were the only false negative prion cases. Two patients without prion disease showed high SI in the striatum and thalamus (Case #18) and cerebral cortex (Case #28) on both FLAIR-T2 and DWI. All the other non-prion patients showed normal SI in the deep grey matter and cerebral cortex on both sequences.
Table 2.
Evaluation of SI in FLAIR-T2 and DWI images of patients with or without prion disease
| Case | FLAIR-T2 | DWI | ||||
|---|---|---|---|---|---|---|
| # | Striatum | Thalamus | Cerebral cortex | Striatum | Thalamus | Cerebral cortex |
| Patients with prion disease | ||||||
| 1 | + | − | − | + | + | − |
| 2 | − | − | − | − | − | − |
| 3 | + | − | − | + | − | + |
| 4 | + | + | − | + | + | − |
| 5 | + | + | + | + | + | + |
| 6 | + | − | + | + | − | + |
| 7 | + | − | − | + | + | − |
| 8 | + | − | + | + | + | + |
| 9 | − | − | − | + | − | + |
| 10 | + | − | − | + | + | − |
| 11 | + | − | + | + | − | + |
| 12 | + | − | + | + | − | + |
| 13 | + | − | − | + | − | + |
| 14 | − | − | − | − | − | − |
| Patients without prion disease | ||||||
| 15 | − | − | − | − | − | − |
| 16 | − | − | − | − | − | − |
| 17 | − | − | − | − | − | − |
| 18 | + | + | − | + | + | − |
| 19 | − | − | − | − | − | − |
| 20 | − | − | − | − | − | − |
| 21 | − | − | − | − | − | − |
| 22 | − | − | − | − | − | − |
| 23 | − | − | − | − | − | − |
| 24 | − | − | − | − | − | − |
| 25 | − | − | − | − | − | − |
| 26 | − | − | − | − | − | − |
| 27 | − | − | − | − | − | − |
| 28 | − | − | + | − | − | + |
| 29 | − | − | − | − | − | − |
SI was evaluated in the striatum, thalamus and cerebral cortex.
+ = hyperintensity; − = normal SI.
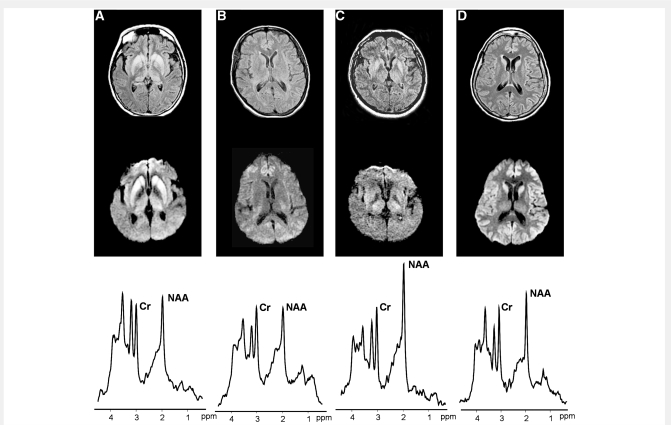
Figure 3.
FLAIR-T2 (top) and DWI (middle), and thalamic 1H-MRS (bottom) from Case #4 with definite sporadic CJD, VV2 (A), Case #2 with definite sporadic fatal insomnia, MM2 (B), Case #18 with possible autoimmune encephalitis (C) and Case #6 with definite sporadic CJD, MM1 (D). On MRI, Case #4 shows increased SI in the striatum and thalamus, Case #2 no SI changes, Case #18 increased SI in the striatum and thalamus and Case #6 increased SI in the striatum and cerebral cortex. On 1H-MRS of the thalamus, all the three prion patients (A, B and D) showed, relative to creatine-phosphocreatine (Cr) a severe reduction of the neuronal marker NAA. The patient without prion disease (C) showed normal thalamic spectrum. ppm = parts per million.
Single voxel 1H-MRS was performed in four regions of interest: thalamus, cerebellum, striatum and occipital cortex (Fig. 2). The mean line width of the water resonance of the subjects included in the study was 6.77 ± 1.99 (SD) Hz in the striatum compared with 3.95 ± 0.99 in the thalamus, 4.25 ± 0.90 in the cerebellum and 3.62 ± 0.58 in the occipital cortex. The higher widths in the striatum resulted in the exclusion (estimated fitting error >20%) of spectra from the striatum of four prion patients, three non-prion patients and two controls. myo-inositol to Cr ratios from the striatum, cerebellum and occipital cortex of one non-prion patient and from the striatum of one control were also excluded from the analysis due to an estimated fitting error >20% for myo-inositol.
The most significant neuro-metabolic group differences were detected in the thalamus and cerebellum (Table 3). In the thalamus of prion patients, post hoc testing (Fig. 3 and Table 4) revealed a reduction in NAA/Cr and NAA/myo-inositol, and an elevation of myo-inositol/Cr, compared with both non-prion and control subjects. Non-prion patients, compared with controls, showed a lower thalamic NAA/Cr. In the two groups of patients, NAA/Cr in the striatum were similarly reduced compared with controls. In the cerebellum of prion patients, NAA/Cr and NAA/myo-inositol were reduced compared with controls.
Table 3.
1H-MRS data obtained in four different regions of interest from patients with prion disease, patients without prion disease and healthy controls
| Regions of interest | NAA/Cr | Cho/Cr | mi/Cr | NAA/mi |
|---|---|---|---|---|
| Thalamus | ||||
| Patients with prion disease (14) | 0.98 ± 0.13 | 0.30 ± 0.07 | 1.05 ± 0.19 | 0.96 ± 0.21 |
| Patients without prion disease (15) | 1.24 ± 0.11 | 0.31 ± 0.05 | 0.86 ± 0.23 | 1.64 ± 0.84 |
| Healthy subjects (10) | 1.37 ± 0.12 | 0.31 ± 0.04 | 0.82 ± 0.15 | 1.71 ± 0.25 |
| P (ANOVA) | <0.001 | 0.939 | 0.014 | 0.002 |
| Striatum | ||||
| Patients with prion disease (10) | 0.85 ± 0.16 | 0.28 ± 0.04 | 0.86 ± 0.29 | 0.97 ± 0.33 |
| Patients without prion disease (12) | 0.87 ± 0.17 | 0.27 ± 0.05 | 0.89 ± 0.391 | 1.27 ± 0.801 |
| Healthy subjects (8) | 1.09 ± 0.24 | 0.24 ± 0.07 | 0.73 ± 0.082 | 1.55 ± 0.322 |
| P (ANOVA) | 0.021 | 0.431 | 0.567 | 0.152 |
| Cerebellum | ||||
| Patients with prion disease (14) | 0.85 ± 0.22 | 0.25 ± 0.04 | 0.72 ± 0.14 | 1.23 ± 0.38 |
| Patients without prion disease (15) | 0.99 ± 0.10 | 0.27 ± 0.06 | 0.65 ± 0.133 | 1.58 ± 0.363 |
| Healthy subjects (10) | 1.15 ± 0.16 | 0.27 ± 0.04 | 0.65 ± 0.12 | 1.71 ± 0.31 |
| P (ANOVA) | 0.001 | 0.411 | 0.283 | 0.014 |
| Occipital cortex | ||||
| Patients with prion disease (14) | 1.19 ± 0.16 | 0.17 ± 0.03 | 0.82 ± 0.16 | 1.51 ± 0.42 |
| Patients without prion disease (15) | 1.26 ± 0.14 | 0.19 ± 0.03 | 0.72 ± 0.143 | 1.89 ± 0.923 |
| Healthy subjects (10) | 1.32 ± 0.07 | 0.19 ± 0.02 | 0.78 ± 0.11 | 1.71 ± 0.21 |
| P (ANOVA) | 0.071 | 0.218 | 0.187 | 0.298 |
Data are expressed as mean ± SD. ANOVA test: P < 0.05 were considered significant. The number of spectra included in the analysis is indicated in parenthesis. When individual metabolites were not included in the analysis (SD > 20%) superscript numbers are used: 1n = 11; 2n = 7; 3n = 14; mi = myo-inositol.
Significant P values are indicated in bold.
Table 4.
Post hoc analysis of 1H-MRS variables that were significant on the ANOVA test (Table 3)
| Prion patients versus non-prion patients | Prion patients versus healthy subjects | Non-prion patients versus healthy subjects | |
|---|---|---|---|
| Thalamus | |||
| NAA/Cr | <0.001 | <0.001 | 0.038 |
| mi/Cr | 0.045 | 0.028 | NS |
| NAA/mi | 0.006 | 0.007 | NS |
| Striatum | |||
| NAA/Cr | NS | 0.034 | 0.048 |
| Cerebellum | |||
| NAA/Cr | NS | <0.001 | NS |
| NAA/mi | NS | 0.035 | NS |
The Student's t-test after Bonferroni correction for multiple comparisons was used, applying a significance threshold of P < 0.05; mi = myo-inositol; NS = not significant.
Differential diagnosis between patients with and without prion disease
The initial diagnosis, based on clinical and EEG findings, of probable prion disease showed a sensitivity of 29% and a specificity of 87%. Seventy-one percent of prion patients and 87% of the patients without prion disease were initially diagnosed as possible prion disease. The 14-3-3 protein test in CSF showed a diagnostic sensitivity, specificity, NPV and PPV of 86, 87, 86 and 87%, respectively, with the number of cases correctly diagnosed equal to 86% (Table 5).
Table 5.
Sensitivity, specificity, predictive values and percentage of cases correctly diagnosed of 1H-MRS variables from the thalamus and cerebellum, DWI and CSF 14-3-3 protein for the discrimination between patients with and without prion disease
| 1H-MRS—thalamus (cutoff) | NAA/Cr ≤1.21 | NAA/mi ≤1.05 |
| Sensitivity (%) | 100 | 79 |
| Specificity (%) | 73 | 100 |
| PPV (%) | 78 | 100 |
| NPV (%) | 100 | 83 |
| Cases correctly identified (%) | 86 | 90 |
| 1H-MRS—cerebellum (cutoff) | NAA/Cr ≤0.88 | NAA/mi ≤1.53 |
| Sensitivity (%) | 64 | 86 |
| Specificity (%) | 87 | 57 |
| PPV (%) | 82 | 67 |
| NPV (%) | 72 | 80 |
| Cases correctly identified (%) | 76 | 62 |
| DWI | CSF 14-3-3 | |
| Sensitivity (%) | 86 | 86 |
| Specificity (%) | 87 | 87 |
| PPV (%) | 86 | 86 |
| NPV (%) | 87 | 87 |
| Cases correctly identified (%) | 86 | 86 |
For 1H-MRS variables cutoff values were determined by receiver operating characteristic curve analysis; mi = myo-inositol.
Considering the receiver operating characteristic curve analysis (Table 5), thalamic 1H-MRS variables showed the greatest ability to differentiate between patients with and without prion disease. In particular, NAA/Cr showed a 100% sensitivity and 100% NPV, and NAA/myo-inositol a 100% specificity and 100% PPV in differentiating prion from non-prion patients. Cerebellar 1H-MRS variables showed, compared with thalamic 1H-MRS, a much lower differential diagnostic power (Table 5).
Diffusion-weighted imaging showed a diagnostic sensitivity, specificity, NPV and PPV of 86, 87, 86 and 87%, respectively; identical to that found for the 14-3-3 protein (Table 5).
The percentages of correct diagnoses were comparable for thalamic NAA/Cr (86%), thalamic NAA/myo-inositol (90%) and diffusion-weighted imaging (86%) and for CSF 14-3-3 protein (86%). The two false negative prion cases on diffusion-weighted imaging (Cases #2 and #14) were correctly diagnosed using thalamic NAA/Cr and NAA/myo-inositol, and the four false positive non-prion patients based on thalamic NAA/Cr values (Cases #19, #24, #27 and #28) were correctly diagnosed as non-prion by diffusion-weighted imaging (#19, #24 and #27) and/or thalamic NAA/myo-inositol (Cases #19, #24, #27 and #28). When the three patients classified as prion patients without a post-mortem or genetic confirmation of the diagnosis (Cases #5, #11 and #12, Table 1) were excluded from the analysis, the percentages of correct diagnoses was similar: thalamic NAA/Cr (85%), thalamic NAA/myo-inositol (90%), and diffusion-weighted imaging (85%) and for CSF 14-3-3 protein (85%).
Logistic regression analysis of thalamic NAA/Cr, thalamic NAA/myo-inositol, diffusion-weighted imaging, CSF 14-3-3 protein and EEG results showed that the only variables relevant to the diagnostic outcome were either thalamic NAA/Cr (P = 0.033, OR = 0.00, 95% CI 0.00–0.25, regression coefficient ± SEM = −17.72 ± 8.33) and diffusion-weighted imaging (P = 0.038, OR = 66.09, 95% CI 1.27–3436.26, regression coefficient ± SEM = 4.19 ± 2.02) or thalamic NAA/Cr (P = 0.034, OR = 0.00, 95% CI 0.00–0.27, regression coefficient ± SEM = –17.74 ± 8.38) and CSF 14-3-3 protein (P = 0.038, OR = 63.80, 95% CI 1.24–3262.15, regression coefficient ± SEM = 4.15 ± 2.01). The combination of thalamic NAA/Cr and diffusion-weighted imaging or thalamic NAA/Cr and CSF 14-3-3 protein showed the same predictive accuracy (93%) in classifying patients as prion or non-prion.
Discussion
In this study, we evaluated the accuracy of different magnetic resonance modalities such as 1H-MRS, diffusion-weighted imaging and FLAIR-T2, in the diagnosis of prion disease. Based on modified clinical and EEG WHO-1998 criteria (Zeidler et al., 1998), all patients included had an initial diagnosis of possible or probable prion disease. Clinical follow-up, genetic studies and, when available, post-mortem examination were used to classify patients as prion or non-prion. Fourteen out of 29 patients were diagnosed with prion disease. The percentage of correctly diagnosed cases was 86% for diffusion-weighted imaging (hyperintensity in the striatum and/or cerebral cortex), 86% for thalamic NAA/creatine (cutoff ≤1.21), 90% for thalamic NAA/myo-inositol (cutoff ≤1.05) and 86% for CSF 14-3-3 protein. All of the prion disease patients had NAA/Cr ratios ≤1.21 (100% sensitivity and 100% NPV), while all the non-prion patients had NAA/myo-inositol ratios above 1.05 (100% specificity and 100% PPV). Non-linear discriminant analysis showed that the combination of thalamic NAA/Cr and diffusion-weighted imaging was best for correctly classifying patients as prion or non-prion (accuracy 93%).
The present study systematically evaluated, for the first time, the diagnostic value of 1H-MRS in the differential diagnosis of prion disease by assessing the metabolite content in brain regions such as the thalamus, striatum, cerebellum and occipital cortex known to be affected in most subtypes of sporadic prion disease (Parchi et al., 1999). Previous 1H-MRS studies on small numbers of patients with classic or variant CJD have detected a variable distribution of neuro-metabolic changes. In general, reduced NAA content and/or increased myo-inositol were detected, in comparison with healthy subjects, in the basal ganglia, thalamus and cerebral cortex (Bruhn et al., 1991; Graham et al., 1993; Pandya et al., 2003; Lim et al., 2004; Cordery et al., 2006; Fulbright et al., 2006; Waldman et al., 2006).
The most striking neuro-metabolic differences between prion patients and healthy controls, found by our study, were in the thalamus and cerebellum, where metabolite ratios NAA/Cr and NAA/myo-inositol showed a marked reduction compared with healthy subjects: −28 and −44% in the thalamus and −26 and −28% in the cerebellum, respectively. These 1H-MRS changes reflect two main neuropathological features of prion disease: neuronal loss, responsible for the reduction of the neuronal marker NAA (Kantarci et al., 2008) and glial activation, responsible for the increase in the glial marker myo-inositol (Brand et al., 1993). The less severe 1H-MRS changes detected in prion cases compared with healthy controls in the striatum and cerebral cortex probably have two different explanations. Spectroscopic magnetic resonance measurements in the striatum are particularly challenging due to susceptibility effects that resulted in an increased peak line width and reduced the precision of peak quantification. On the other hand, the involvement of cerebral cortex in prion disease is strictly related to the disease subtype (Parchi et al., 1999). In particular, cortical involvement was absent at the time of the MR scan in VV2, MV2 and fatal insomnia (see Cases #1, #2, #4, #7, #10 and #14), which is consistent with previous findings (Tschampa et al., 2007).
The most significant neuro-metabolic differences between prion and non-prion patients were found in the thalamus. In prion compared with non-prion patients, thalamic NAA/Cr was reduced by 21% and NAA/myo-inositol by 41%. Myo-inositol/Cr in prion cases was increased by 22%. Non-prion patients showed, compared with healthy controls, a reduction in NAA/Cr in the thalamus, much milder than that detected in prion patients, but a reduction to a similar degree to the prion cases in the striatum. In addition, non-prion patients showed normal NAA/myo-inositol in the thalamus.
All our suspected prion patients underwent a neuroimaging study that included axial FLAIR-T2 and DWI sequences. In prion patients, these sequences have been reported to be the most sensitive in the detection of the diagnostic increase in SI in the basal ganglia and/or cortex (Collie et al., 2001; Shiga et al., 2004; Tschampa et al., 2005; Kallenberg et al., 2006; Macfarlane et al., 2007). Diffusion-weighted imaging showed increased SI in the striatum and/or in the cortex of all prion patients except Cases #2 and #14. Increased SI on DWI was present in the striatum in 86% of cases whereas increased cortical SI was detected in 57%. FLAIR images showed a SI increase in the striatum in 79% of the prion patients and in the cortex in only 42%, confirming the higher sensitivity of DWI compared with FLAIR in detecting increased SI in prion disease. Hyperintense regions in DWI images are characterized by reduced values of the water apparent diffusion coefficient (Bahn and Parchi, 1999; Tschampa et al., 2003; Manners et al., 2009). A combined effect of T2 and apparent water coefficient changes are likely to be responsible for the more striking abnormalities seen on DWI compared with FLAIR sequences.
The only DWI false negative Cases (#2 and #14) were sporadic or genetic fatal insomnia cases. These prion disease subtypes are characterized by lack of spongiform changes in the basal ganglia and thalamus and by only late and small cortical spongiform changes (Parchi et al., 1999). It has recently been demonstrated that spongiform changes are the pathological basis underlying increased SI (Manners et al., 2009). Indeed, this was also the case for our sporadic fatal insomnia patient (Case #2) where pathology revealed a severe neuronal loss and gliosis in the thalamus in the absence of significant spongiosis (P. Parchi, personal communication). The absence of increased SI on DWI, associated with the lack of spongiform changes, has previously been reported in one case of sporadic fatal insomnia (Shiga et al., 2004) and in patients with familial fatal insomnia (Montagna et al., 2003; Haik et al., 2008). In both cases of fatal insomnia, thalamic 1H-MRS showed a severe reduction in NAA/Cr confirming the early and severe neuronal loss, and, in the patient with sporadic fatal insomnia. an increase in myo-inositol/Cr indicating glial proliferation 11 months before death. These findings are consistent with a recent combined 1H-MRS and pathological study of a patient with familial fatal insomnia where marked thalamic in vivo increase in myo-inositol content was associated with severe gliosis at post-mortem (Haik et al., 2008).
The largest available MRI studies of CJD patients are retrospective, and used mainly T2- and proton density-weighted images to evaluate SI in the caudate nucleus and putamen (Tschampa et al., 2005; Collins et al., 2006). Tschampa and colleagues studied 193 suspected sporadic CJD patients and found a diagnostic sensitivity ranging from 58.3% to 70.8%, depending on the rater, and a specificity >80%. A much lower diagnostic sensitivity was reported by Collins and colleagues in a larger multi-centre study that found the typical striatial hyperintensity in only 39.1% of 1036 definite CJD patients. The low utilization of more recent and sensitive pulse sequences, such as FLAIR and DWI, and the lack of evaluation of cerebral cortical signal are the most likely explanations of the much lower sensitivity and specificity demonstrated in these large studies compared with ours and other smaller, more recent MRI studies utilizing FLAIR-T2 and DWI sequences, which all showed a sensitivity and specificity ∼90% (Shiga et al., 2004; Young et al., 2005).
Several of our prion patients presented an increased SI in the thalamus on DWI (43%). All of our patients with increased SI in the thalamus also showed high SI in the striatum. Previous DWI studies have shown a lower detection of increased SI in the thalamus of CJD patients (Shiga et al., 2004; Young et al., 2005; Kallenberg et al., 2006) ranging from 12.5% (3 out of 26, Shiga et al., 2004) to 34% (14 out of 40, Young et al., 2005). The higher percentage of thalamic abnormalities in our study may be related to the presence, in our CJD group, of a prevalence of VV2 and MV2 subtypes, both characterized by a severe pathological thalamic involvement (Parchi et al., 1999) and high frequency of increased SI on DWI/T2-weighted images (Krasnianski et al., 2006; Heinemann et al., 2007). Indeed all our CJD patients with high thalamic SI were VV2 or MV2 subtypes. As previously reported, none of our MM1 patients showed increased thalamic SI (Krasnianski et al., 2006; Heinemann et al., 2007).
Only two non-prion patients presented increased SI on FLAIR and DWI in the striatum and thalamus (Case #18) and cerebral cortex (Case #28). Case #18, who presented normal thalamic NAA/Cr and NAA/myo-inositol, was affected by encephalitis that was probably due to autoimmunity against deep grey matter neurons (Dale et al., 2004). In this patient corticosteroid therapy resulted in a regression of symptoms and signs and a resolution of SI enhancement in both FLAIR and DWI. Case #28 presented with subentrant epileptic seizures at the time of the scan, probably causing the increase in cortical SI. In this patient, CSF 14-3-3 protein was high at the time of the scan but normalized 4 months later, in association with a clinical improvement.
The diagnostic capability of combined DWI and thalamic 1H-MRS to correctly identify prion and non-prion patients was slightly higher than the increase in the 14-3-3 protein in the CSF. Using 14-3-3 protein levels, 86% of patients were correctly classified in our study, in line with previous reports (Collins et al., 2006; Sanchez-Juan et al., 2006). On the other hand, the contribution of EEG to the correct diagnostic classification of our patients was poor compared with other studies, with a sensitivity <30%. This may be due to the prevalence in our study of CJD subtypes (VV2, MV2, thalamic MM2 and familial fatal insomnia) characterized by a relatively mild and late cortical involvement (Parchi et al., 1999).
In conclusion, we combined conventional MRI and 1H-MRS in the differential diagnosis of patients with possible or probable prion disease on the basis of clinical features. Thalamic 1H-MRS (NAA/Cr and NAA/myo-inositol) and DWI showed a very high correct diagnostic classification (86–90%). Most importantly, (i) the combination of 1H-MRS and DWI results could predict prion disease (=PPV) in a patient positive for both increased SI on DWI and reduced thalamic NAA/Cr on 1H-MRS with a likelihood of 100%; (ii) the likelihood of not having a prion disease (=NPV) in a patient with the absence of both SI increase on DWI and NAA/myo-inositol reduction on thalamic 1H-MRS was equal to 100% and (iii) the combination of thalamic NAA/Cr and DWI in a logistic regression analysis resulted in a correct classification of 93% of patients.
This study was performed on a relatively small number of patients. However, it was a prospective study in which, unlike most of the previous studies, the non-prion patients were all recruited on the basis of a clinical presentation of ‘possible’ or ‘probable’ prion disease. This allowed a more correct evaluation of the specificity of the diagnostic magnetic resonance markers.
Although further work on a larger and more varied patient population will be needed, overall our findings indicate that the combination of thalamic 1H-MRS (acquisition duration 10 min) and brain DWI (acquisition duration 2 min) may increase the diagnostic accuracy of the magnetic resonance scan and that both sequences should be included routinely in the clinical work-up of patients with suspected prion disease.
Funding
EU Grants “PRIONMRDIAGNOSTICS” (contract no. QLK4-CT-01763); NEUROPRION (contract no. FOOD-CT-2004-506 579).
Glossary
Abbreviations
- CJD
Creutzfeldt–Jakob disease
- Cr
creatine-phosphocreatine
- CSF
cerebrospinal fluid
- DWI
diffusion-weighted images
- EEG
electroencephalography
- FLAIR
fluid-attenuated inversion recovery
- 1H-MRS
proton magnetic resonance spectroscopy
- MRI
magnetic resonance imaging
- NAA
N-acetyl-aspartate
- NPV
negative predictive value
- PPV
positive predictive value
- SI
signal intensity
References
- Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. 4th. London: Blackwell; 2002. [Google Scholar]
- Bahn MM, Parchi P. Abnormal diffusion-weighted magnetic resonance images in Creutzfeldt-Jakob disease. Arch Neurol. 1999;56:577–83. doi: 10.1001/archneur.56.5.577. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–98. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Bruhn H, Weber T, Thorwirth V, Frahm J. In-vivo monitoring of neuronal loss in Creutzfeldt-Jakob disease by proton magnetic resonance spectroscopy. Lancet. 1991;337:1610–11. doi: 10.1016/0140-6736(91)93309-w. [DOI] [PubMed] [Google Scholar]
- Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF. CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology. 2001;56:1528–33. doi: 10.1212/wnl.56.11.1528. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Colucci M, Xie Z, Zou W, Li C, Parchi P, et al. Sensitivity of 14-3-3 protein test varies in subtypes of sporadic Creutzfeldt–Jakob disease. Neurology. 2004;63:436–42. doi: 10.1212/01.wnl.0000135153.96325.3b. [DOI] [PubMed] [Google Scholar]
- Collie DA, Sellar RJ, Zeidler M, Colchester AC, Knight R, Will RG. MRI of Creutzfeldt-Jakob disease: imaging features and recommended MRI protocol. Clin Radiol. 2001;56:726–39. doi: 10.1053/crad.2001.0771. [DOI] [PubMed] [Google Scholar]
- Collins SJ, Sanchez-Juan P, Masters CL, Klug GM, van Duijn C, Poleggi A, et al. Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt-Jakob disease. Brain. 2006;129:2278–87. doi: 10.1093/brain/awl159. [DOI] [PubMed] [Google Scholar]
- Cordery RJ, MacManus D, Godbolt A, Rossor MN, Waldman AD. Short TE quantitative proton magnetic resonance spectroscopy in variant Creutzfeldt-Jakob disease. Eur Radiol. 2006;16:1692–8. doi: 10.1007/s00330-005-0090-4. [DOI] [PubMed] [Google Scholar]
- Dale RC, Church AJ, Surtees RA, Lees AJ, Adcock JE, Harding B, et al. Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain. 2004;127:21–33. doi: 10.1093/brain/awh008. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Kingsley PB, Guo X, Hoffmann C, Kahana E, Chapman JC, et al. The imaging appearance of Creutzfeldt–Jakob disease caused by the E200K mutation. Magn Reson Imaging. 2006;24:1121–9. doi: 10.1016/j.mri.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Gambetti P, Kong Q, Zou W, Parchi P, Chen SG. 1: Sporadic and familial CJD: classification and characterisation. Br Med Bull. 2003;66:213–39. doi: 10.1093/bmb/66.1.213. [DOI] [PubMed] [Google Scholar]
- Graham GD, Petroff OA, Blamire AM, Rajkowska G, Goldman-Rakic P, Prichard JW. Proton magnetic resonance spectroscopy in Creutzfeldt-Jakob disease. Neurology. 1993;43:2065–8. doi: 10.1212/wnl.43.10.2065. [DOI] [PubMed] [Google Scholar]
- Haïk S, Galanaud D, Linguraru MG, Peoc'h K, Privat N, Faucheux BA, et al. In vivo detection of thalamic gliosis: a pathoradiologic demonstration in familial fatal insomnia. Arch Neurol. 2008;65:545–9. doi: 10.1001/archneur.65.4.545. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Krasnianski A, Meissner B, Gloeckner SF, Kretzschmar HA, Zerr I. Molecular subtype-specific clinical diagnosis of prion diseases. Vet Microbiol. 2007;123:328–35. doi: 10.1016/j.vetmic.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Kallenberg K, Schulz-Schaeffer WJ, Jastrow U, Poser S, Meissner B, Tschampa HJ, et al. Creutzfeldt-Jakob disease: comparative analysis of MR imaging sequences. Am J Neuroradiol. 2006;27:1459–62. [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Knopman DS, Dickson DW, Parisi JE, Whitwell JL, Weigand SD, et al. Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology. 2008;248:210–20. doi: 10.1148/radiol.2481071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnianski A, Bartl M, Sanchez Juan PJ, Heinemann U, Meissner B, Varges D, et al. Fatal familial insomnia: clinical features and early identification. Ann Neurol. 2008;63:658–61. doi: 10.1002/ana.21358. [DOI] [PubMed] [Google Scholar]
- Krasnianski A, Schulz-Schaeffer WJ, Kallenberg K, Meissner B, Collie DA, Roeber S, et al. Clinical findings and diagnostic tests in the MV2 subtype of sporadic CJD. Brain. 2006;129:2288–96. doi: 10.1093/brain/awl123. [DOI] [PubMed] [Google Scholar]
- Lim CC, Tan K, Verma KK, Yin H, Venketasubramanian N. Combined diffusion-weighted and spectroscopic MR imaging in Creutzfeldt-Jakob disease. Magn Reson Imaging. 2004;22:625–9. doi: 10.1016/j.mri.2004.01.042. [DOI] [PubMed] [Google Scholar]
- Macfarlane RG, Wroe SJ, Collinge J, Yousry TA, Jäger HR. Neuroimaging findings in human prion disease. J Neurol Neurosurg Psychiatry. 2007;78:664–70. doi: 10.1136/jnnp.2006.094821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners DN, Parchi P, Tonon C, Capellari S, Strammiello R, Testa C, et al. Pathological correlates of diffusion MRI changes in Creutzfeldt-Jakob disease. Neurology. 2009;72:1425–31. doi: 10.1212/WNL.0b013e3181a18846. [DOI] [PubMed] [Google Scholar]
- Montagna P, Gambetti P, Cortelli P, Lugaresi E. Familial and sporadic fatal insomnia. Lancet Neurol. 2003;2:167–76. doi: 10.1016/s1474-4422(03)00323-5. [DOI] [PubMed] [Google Scholar]
- Pandya HG, Coley SC, Wilkinson ID, Griffiths PD. Magnetic resonance spectroscopic abnormalities in sporadic and variant Creutzfeldt-Jakob disease. Clin Radiol. 2003;58:148–53. doi: 10.1053/crad.2002.1080. [DOI] [PubMed] [Google Scholar]
- Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol. 1999;46:224–33. [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Sanchez-Juan P, Green A, Ladogana A, Cuadrado-Corrales N, Sáanchez-Valle R, Mitrováa E, et al. CSF tests in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology. 2006;67:637–43. doi: 10.1212/01.wnl.0000230159.67128.00. [DOI] [PubMed] [Google Scholar]
- Schröter A, Zerr I, Henkel K, Tschampa HJ, Finkenstaedt M, Poser S. Magnetic resonance imaging in the clinical diagnosis of Creutzfeldt-Jakob disease. Arch Neurol. 2000;57:1751–7. doi: 10.1001/archneur.57.12.1751. [DOI] [PubMed] [Google Scholar]
- Shiga Y, Miyazawa K, Sato S, Fukushima R, Shibuya S, Sato Y, et al. Diffusion-weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt-Jakob disease. Neurology. 2004;63:443–9. doi: 10.1212/01.wnl.0000134555.59460.5d. [DOI] [PubMed] [Google Scholar]
- Steinhoff BJ, Zerr I, Glatting M, Schulz-Schaeffer W, Poser S, Kretzschmar HA. Diagnostic value of periodic complexes in Creutzfeldt-Jakob disease. Ann Neurol. 2004;56:702–8. doi: 10.1002/ana.20261. [DOI] [PubMed] [Google Scholar]
- Tschampa HJ, Mürtz P, Flacke S, Paus S, Schild HH, Urbach H. Thalamic involvement in sporadic Creutzfeldt–Jakob disease: a diffusion-weighted MR imaging study. AJNR Am J Neuroradiol. 2003;24:908–15. [PMC free article] [PubMed] [Google Scholar]
- Tschampa HJ, Zerr I, Urbach H. Radiological assessment of Creutzfeldt-Jakob disease. Eur Radiol. 2007;17:1200–11. doi: 10.1007/s00330-006-0456-2. [DOI] [PubMed] [Google Scholar]
- Tschampa HJ, Kallenberg K, Urbach H, Meissner B, Nicolay C, Kretzschmar HA, et al. MRI in the diagnosis of sporadic Creutzfeldt–Jakob disease: a study on inter-observer agreement. Brain. 2005;128:2026–33. doi: 10.1093/brain/awh575. [DOI] [PubMed] [Google Scholar]
- Waldman AD, Cordery RJ, MacManus DG, Godbolt A, Collinge J, Rossor MN. Regional brain metabolite abnormalities in inherited prion disease and asymptomatic gene carriers demonstrated in vivo by quantitative proton magnetic resonance spectroscopy. Neuroradiology. 2006;48:428–33. doi: 10.1007/s00234-006-0068-1. [DOI] [PubMed] [Google Scholar]
- Young GS, Geschwind MD, Fischbein NJ, Martindale JL, Henry RG, Liu S, et al. Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: high sensitivity and specificity for diagnosis. Am J Neuroradiol. 2005;26:1551–62. [PMC free article] [PubMed] [Google Scholar]
- Zeidler M, Gibbs CJ, Jr, Meslin F. WHO manual for strengthening diagnosis and surveillance of Creutzfeldt-Jakob disease. Geneva: World Health Organization; 1998. pp. 47–51. [Google Scholar]
- Zerr I, Pocchiari M, Collins S, Brandel JP, de Pedro Cuesta J, Knight RS, et al. Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt–Jakob disease. Neurology. 2000;55:811–15. doi: 10.1212/wnl.55.6.811. [DOI] [PubMed] [Google Scholar]