Abstract
Escherchia coli MutY plays an important role in preventing mutations associated with the oxidative lesion 7,8-dihydro-8-oxo-2′-deoxyguanosine (OG) in DNA by excising adenines from OG:A mismatches as the first step of base excision repair. To determine the importance of specific steps in the base pair recognition and base removal process of MutY, we have evaluated the effects of modifications of the OG:A substrate on the kinetics of base removal, mismatch affinity and repair to G:C in an Escherchia coli-based assay. Surprisingly, adenine modification was tolerated in the cellular assay, while modification of OG results in minimal cellular repair. High affinity for the mismatch and efficient base removal require the presence of OG. Taken together, these results suggest that the presence of OG is a critical feature for MutY to locate OG:A mismatches and select the appropriate adenines for excision to initiate repair in vivo prior to replication.
The mismatch repair (MMR) pathway in E. coli relies on methylation at a GATC sequence to direct the repair machinery to remove the mismatched base on the newly synthesized strand.1 However, almost two decades ago, an activity was detected in E. coli cell extracts that restored G:A mismatches to G:C matches and was insensitive to the methylation state of the template strand.2–4 Concurrently, a mutator locus in E. coli (mutY) was identified that generated G:C → T:A transversion mutations.5 It was later determined that the mutY gene product is an adenine glycosylase capable of removing adenines from G:A mismatches as the first step in base excision repair (BER).6–10 The subsequent activity of downstream BER pathway enzymes, e.g. the AP endonuclease, deoxyribophosphate lyase, polymerase and ligase, restores the G:C base pair in a methylation-independent fashion.11 MutY was also found to participate in the prevention of mutations caused by 7,8-dihydro-8-oxo-2′-deoxyguanosine (OG, 1) by removal of adenine from OG:A mismatches.10,12,13,14 Polymerase misinsertion of dAMP opposite OG creates OG:A mismatches that can lead to formation of T:A base pairs upon a second round of replication.12 Recently, MutY has been in the spotlight due to the correlation between inherited biallelic mutations in the gene encoding the human homologue of MutY (MUTYH) and colorectal cancer.10,15,16
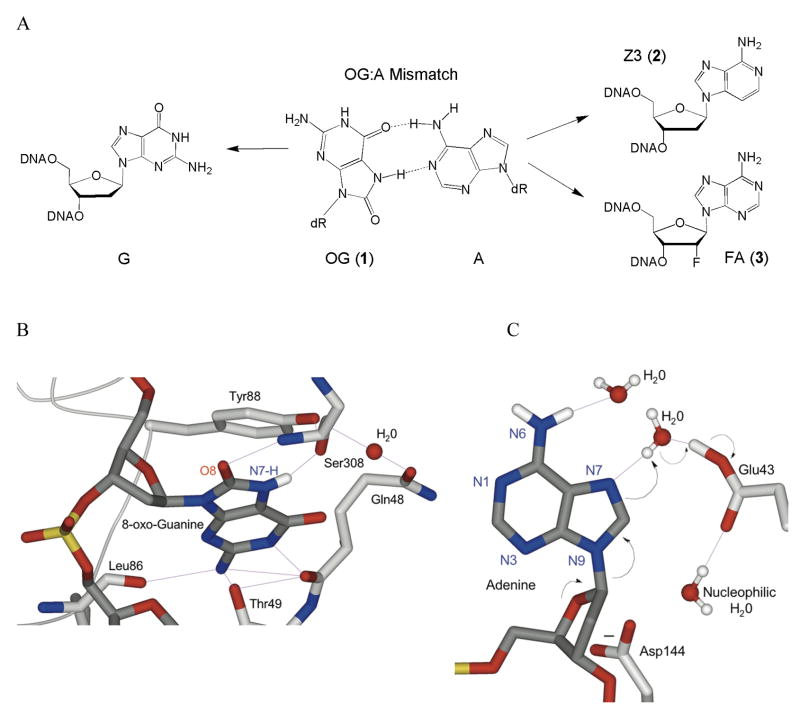
We have previously examined the features of mismatched substrates that are required for efficient lesion recognition and adenine excision by MutY using pre-steady state and single-turnover kinetics.17–19 Pre-steady state experiments revealed that MutY has a high affinity for the product such that release of the DNA product is rate-limiting.17,19,20 Moreover, the identity of the base opposite A greatly affects both the rate of product release as well as the intrinsic rate of adenine removal determined under single-turnover conditions.17 Product release is likely enhanced in vivo by interactions with other BER enzymes.21–23 Functional studies have been complemented by X-ray structural studies of an inactive variant of Bacillus stearothermophilus MutY (D144N BsMY) bound to an OG:A mismatch (referred to as the lesion recognition complex or LRC)24 that show that there is significant reorganization of the OG:A mismatch: the OG was found to be within the helix but swiveled from the OGsyn conformation to the anti conformation, while the A nucleotide was flipped-out of the helix and embedded in a protein pocket (Fig. 1).24
Figure 1. Recognition of an OG:A mismatch by D144N BsMY and modifications made to probe features of mismatch repair.
(a) Structure of an OG:A base pair and the substitutions made of the mismatch that were examined (b and c) Close-up views of recognition of OG (b) and A (c) from the X-ray crystal structure of D144N Bacillus stearothermophilus MutY bound to an OG:A mismatch-containing duplex. In both panels, portions of the BsMY backbone and important residues are shown in light gray, while DNA is rendered in dark gray. Atoms are colored red (oxygen), blue (nitrogen), and gold (phosphorus), while implied protons are white. Hydrogen bonding interactions are shown as purple lines. Panel B highlights the first step of the proposed dissociative mechanism of MutY, with black arrows representing the flow of electrons. Asn144 is represented as the natural Asp144 to emphasize its proposed role in stabilizing an oxocarbenium ion intermediate. Images generated from pdb file 1RRQ from the World Wide Protein Data Bank, based on data from ref. 24.
Structural and functional studies have elucidated aspects of MutY and its substrates that are important for recognition and catalysis; however, the question of how specific defects in mismatch recognition and adenine excision impact overall repair in a cellular context remains unanswered. Thus, to determine the importance of various steps in the recognition and removal process mediated by MutY, we have evaluated specific modifications of an OG:A substrate both in vitro, using adenine glycosylase and binding assays, and in vivo, using a cellular repair assay. Specifically, the OG:A substrate was modified to alter OG by replacement with G, and A by replacement with 3-deaza-2′-deoxyadenosine (Z3, 2) (Fig. 1). The replacement of OG was made to test the importance of the 8-oxo group. The Z3 replacement for A was chosen as a subtle modification of A that should not significantly affect base pair formation, but may reduce the efficiency of base excision by altering the electronic properties of the target base. Indeed, previous work in our laboratories using hydrophobic isosteres of A have implicated the adenine N3 as important for catalysis of base removal by MutY.18
Modifications of both bases in the OG:A bp result in a reduced rate of adenine removal under single-turnover conditions; however, only replacement of OG with G significantly impacts mismatch repair in the cellular assay. Indeed, minimal repair of the G:A substrate was mediated by MutY. Interestingly, the impact of deviations from OG:A on repair correlates most closely with the extent of decreased affinity relative to OG:A. Replacement of A with Z3 does not significantly affect the affinity of MutY for the mismatch, while replacement of OG dramatically reduced DNA binding affinity. To test the importance of the glycosylase function of MutY, the repair of a noncleavable 2′-deoxyadenosine analogue, 2′-deoxy-2′-fluoroadenosine (FA, 3), opposite OG and G was also examined. Only a small amount of repair of the OG:FA base pair appears to be mediated by MutY suggesting that adenine excision is essential. Importantly, however, the results with the Z3 analogue show that a reduced rate of base excision does not similarly reduce the overall extent of repair. Taken together, this work suggests that a critical step for repair mediated by MutY in vivo is locating OG and intercepting the mismatch prior to replication.
RESULTS
Glycosylase activity of MutY with modified substrates
To assess the effect of the alterations of the OG:A mismatch on the glycosylase activity of MutY, the rates of base excision were determined using two different 30 bp duplex DNA substrates (Table 1). Duplex 1 contains a centrally located mismatch in the BmtI sequence context used in the cellular assay. Duplex 2 is the duplex sequence that we have used extensively in analyzing the glycosylase activity of MutY.17 In the series of experiments described herein, the glycosylase activity was also assayed using two NaCl concentrations, 30 mM and 150 mM, in the reaction buffer.
Table 1.
Rate constants (k2) and dissociation constants (Kd) for MutY with duplex DNA containing substrates or substrate analogues
| Sequence | Central base-pair | k2 (min−1)a | Kd (nM)b | |
|---|---|---|---|---|
| 30 mM NaCl | 150 mM NaCl | |||
| Duplex 1 | OG:A | 15 ± 3 | 12 ± 1 | NDc |
| OG:Z3 | 0.08 ± 0.01 | 0.10 ± 0.02 | ND | |
| G:A | 0.26 ± 0.04 | < 0.007e | ND | |
| G:Z3 | 0.02 ± 0.01 | MCd | ND | |
| Duplex 2 | OG:A | 12 ± 2f | 12 ± 3g | < 0.04h |
| OG:Z3 | 0.11 ± 0.01 | 0.12 ± 0.01 | < 0.01 | |
| G:A | 1.6 ± 0.2f | 0.17 ± 0.02 | 21 ± 4h | |
| G:Z3 | 0.09 ± 0.01 | 0.02 ± 0.01 | 44 ± 4 | |
Rate constants for WT MutY measured at 37 °C under single-turnover conditions. All rate constants represent mean values ± S.D. and at least three separate experiments.
Dissociation constants for E37S MutY measured at 25 °C All Kd values represent mean values ± SD and at least three separate experiments.
ND = Values not measured for this duplex
MC = Minimal cleavage; less than 1 % product observed above background
This value is an estimated upper limit based on the extent of cleavage observed at 90 min.
Values reported previously.17
Value reported previously.36
Values reported previously.25
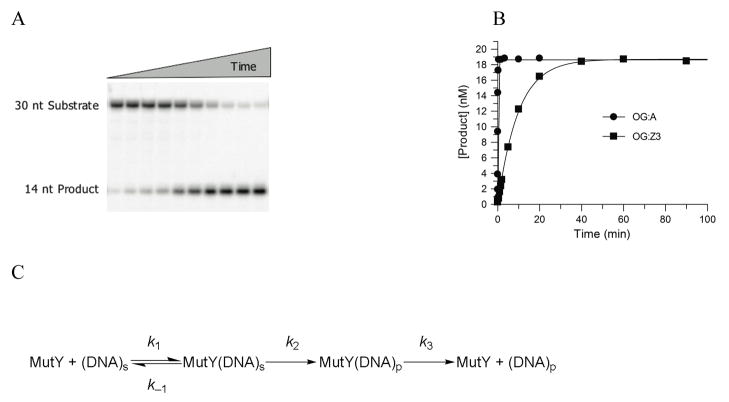
The glycosylase activity of MutY is monitored by measuring the extent of strand scission that occurs at the abasic site produced by the base removal activity of MutY upon quenching the reaction with NaOH (Fig. 2a,b). Analysis of product formation under multiple-turnover conditions ([MutY] < [DNA]) with both duplex OG:A and OG:Z3-containing substrates showed biphasic behavior characterized by an exponential burst of product formation, followed by a slow linear steady-state formation of product. This biphasic profile is due to slow release of MutY from the DNA product.17,20 Based on this behavior the same minimal kinetic scheme and approach to determine the relevant rate constants as we have described previously was used (Fig. 2c).17 Of note, the rate constant k2 was determined under conditions of single-turnover, where [DNA] < [MutY] (Fig. 2).
Figure 2. Kinetic Analysis of the Glycosylase Activity of MutY.
(a) A representative storage phosphor autoradiogram of the PAGE analysis of strand scission at Z3 (creating 14 nt fragment) upon treatment of the OG:Z3-containing duplex (duplex 2) with MutY at 30 mM NaCl, followed by NaOH quenching. (b) Plotting of the data from panel A, with comparison to that obtained with an OG:A-containing duplex 2 under the same conditions. The data are fitted to a single-exponential to determine the rate constant, k2. (c) Minimal kinetic scheme used for analysis of MutY glycosylase activity
The rate constant k2 for adenine removal by MutY with duplex substrates containing OG:A is nearly identical in both sequence contexts and at both low and high salt concentrations (Table 1). Replacement of A with Z3 significantly reduced the rate constant k2 for Z3 excision, with both OG:Z3-containing substrates by approximately 100-fold compared to that determined for removal of A from the corresponding OG:A substrate (Fig. 2b and Table 1). Similar to the processing of the OG:A substrates, the base removal activity of MutY with OG:Z3 substrates is not significantly affected by either the sequence context or the reaction buffer salt concentration. With the OG:A-containing substrate, the rate constant k3, related to product release, is also not affected by the sequence context, but is increased approximately 10-fold by the higher salt concentration (at 150 mM NaCl, k3 = 0.03 ± 0.01 min−1) compared to the value determined at lower salt (at 30 mM NaCl, k3 = 0.004 ± 0.002 min−1).17 The rate constant k3 measured for the reaction of MutY with the OG:Z3 substrate was essentially identical to that measured with the OG:A substrate under all conditions. This is not unexpected since k3 is dominated by release of MutY from the DNA product,20 which is the same for both substrates after base excision and release.
Analysis of the base removal activity of MutY with the G:A or G:Z3 mismatch-containing duplexes shows that the processing of these substrates is sensitive to sequence context and the buffer salt concentration (Table 1). In duplex 1, the rate constant k2 with the G:A substrate at 30 mM NaCl (0.26 ± 0.04 min−1) represents a 50-fold reduction compared to rate with the corresponding OG:A substrate. The magnitude of the preference for OG:A over G:A with duplex 1 is greater than with duplex 2 (50-fold versus 10-fold) due to the reduced rate constant for G:A in duplex 1 compared to duplex 2.17,25 The higher salt concentration had an even more dramatic effect on the reaction of MutY with G:A substrates. In duplex 2 at 150 mM NaCl, the rate constant k2 for adenine removal with the G:A substrate is reduced by approximately 70-fold compared to the value with the OG:A duplex. In the case of duplex 1, where the sequence context has already reduced the rate of adenine removal, the further reduction caused by the high salt concentration results in minimal adenine removal such that only an estimate of the rate constant could be obtained (< 0.007 min−1). The replacement of A with Z3 in G:A mismatches also reduces the rate of base removal by MutY. This is most evident at the lower salt concentrations in both sequence contexts, where the magnitude of the reduction is approximately 13- and 17-fold in duplex 1 and duplex 2, respectively. A biphasic production curve under multiple-turnover conditions for the reaction of MutY with the G:A and G:Z3 substrates at 150 mM NaCl and with duplex 1 was not observed. This is likely due to the reduction in k2 such that it becomes similar to k3, which consequently makes determining k3 difficult using this approach.
MutY Binds Tightly to OG:Z3-containing duplexes
An inactive variant of MutY (E37S MutY) that retains high substrate affinity18 was used in electrophoretic mobility shift assays (EMSA) to determine dissociation constants (Kd) of MutY with the modified substrates (Table 1). E37S MutY exhibits a strong preference for binding OG-containing duplexes over G-containing duplexes when paired with A. The apparent dissociation constant is so small with OG-containing duplexes that only an upper limit could be determined (Kd < 0.04 nM) for the OG:A duplex versus Kd of 21 ± 4 nM for the G:A duplex. Notably, this represents a > 500-fold preference of E37S MutY for the OG duplex over the G-counterpart. E37S MutY was found to also bind with similarly high affinity to the OG:Z3-containing DNA duplex (Kd < 0.01 nM). The binding affinity of MutY for G:Z3 was reduced over 4000-fold, with a Kd value of 44 ± 4 nM. Thus, the dissociation constants demonstrate that alteration of the adenine base by replacement of the nitrogen at N3 with carbon to form Z3 does not significantly alter the affinity of MutY when paired opposite OG or G. However, when paired with either A or Z3, E37S MutY exhibits a significantly higher affinity for duplexes containing OG over G. This illustrates that the substrate affinity is dominated by the presence of OG.
Cellular Assay Design and Substrate Preparation
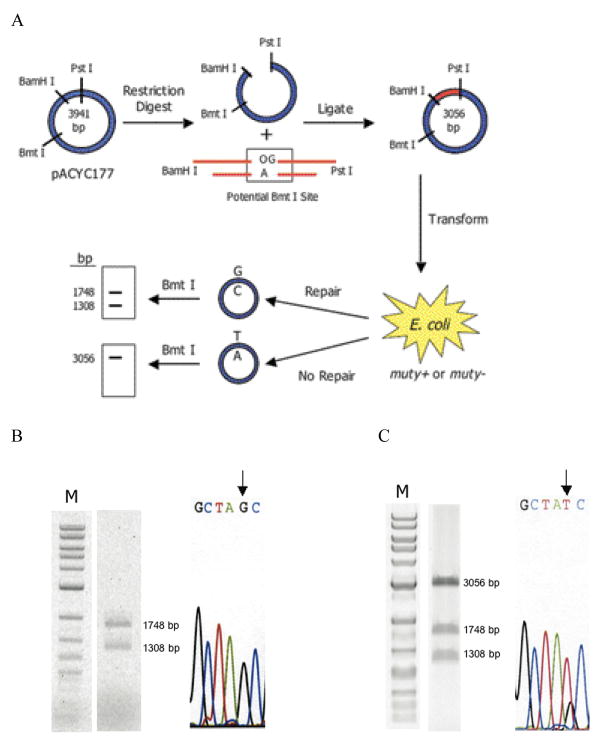
To allow for direct comparison between glycosylase activity and the cellular repair of a given base pair, we devised a strategy (Fig. 3a) that takes advantage of the ability to enzymatically ligate a DNA duplex containing the base pair of interest into a circular DNA plasmid. Repair initiated by MutY at the inserted base pair creates a restriction site that can be analyzed by restriction fragment analysis of the recovered amplified plasmid. This assay is similar to that reported for analysis of mutagenesis and repair of clustered DNA damage.26,27
Figure 3. Cell-based MutY-mediated repair assay.
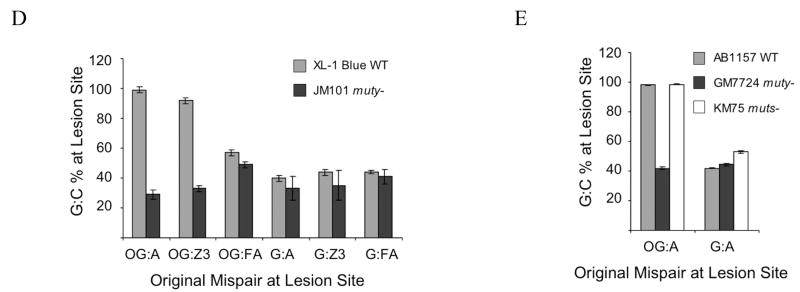
(a) Schematic representation of the assay. A mismatch-containing oligonucleotide is ligated into a plasmid vector and transformed into E. coli (mutY+ or mutY−). Repair or correct replication of the mismatch generates a G:C bp that creates a second Bmt I restriction site. The amount of G:C bp is determined via restriction fragment analysis of the recovered plasmid. The amount of repair mediated by MutY is determined by the % G:C bp created in cells containing MutY relative to those lacking MutY. (b and c) Restriction fragment and sequence analysis of lesion-containing plasmids rescued from E. coli. Representative data depicting initial plasmid containing an OG:A mismatch that has been completely converted to G:C (b). and initial plasmid containing a G:A mismatch that has not been converted completely to G:C (c). Gels shown are EtBr-stained agarose (M = 1 kb DNA ladder). Sequencing portion shown is of the BmtI recognition sequence and the arrow points to the location of the lesion. Levels of repair (percent G:C at lesion site) mediated by MutY are assessed by quantification of the agarose gels in which the percent G:C represents an average of at least three separate experiments in which > 100 colonies were pooled and amplified for the digestion analysis. (d and e) Summary graph of percent G:C bp determined from restriction fragment analysis of various base pair mismatches in E coli strains XL-1 Blue and JM101 mutY− (d) and AB1157, GM7724, and KM75 (e). Repair is related to the amount of G:C base pairs produced at the lesion site determined by BmtI restriction fragment analysis of mismatch-containing plasmid isolated from E. coli possessing MutY versus lacking MutY. Data represent mean values and error bars represent standard deviation of the sample set, which include at least three separate experiments.
Specifically, a DNA duplex (Duplex 3) containing a centrally located O:Y mismatch (where O = OG or G, Y = A, Z3 or FA) and the appropriate ends was ligated into a plasmid vector (pACYC177). Upon repair of the O:Y mismatch to a G:C base pair, a second BmtI restriction site is created in addition to the one already present in the cloning vector. The lesion containing plasmid is transformed into the appropriate E. coli strain. Subsequent amplification and isolation of the plasmid, restriction digestion using BmtI, and agarose gel electrophoresis produces one band at 3056 bps if G:C has not been restored at the lesion site, and two bands at 1748 and 1308 bps if the second BmtI recognition sequence has been created. In addition, DNA sequence analysis provides data complementary to the restriction fragment analysis to determine the identity of the base pair at the original mismatch site. The extent that repair is mediated by MutY is assessed by comparing mutY+ versus mutY− E. coli strains.
Endogenous MutY can Repair OG:A, but not G:A, in vivo
Using the outlined approach, plasmids originally containing OG:A and G:A substrates were isolated from XL-1 Blue E. coli containing endogenous MutY. With an OG:A mismatch, digestion by BmtI (Fig. 3b) shows the presence of only two bands (1748 and 1308 bps), indicating the generation of a G:C base pair and complete repair of the mismatch by bacterial BER in the E. coli. DNA sequence analysis confirms the presence of only G:C at the lesion site (Fig. 3b). Interestingly, in this same E. coli strain, the restriction fragment analysis (Fig. 3c) shows that a G:A mismatch is not fully converted to G:C as indicated by the large intensity of the 3056 bp fragment. DNA sequence analysis shows a significant amount of both T and G bases present at the lesion site, indicating that T:A transversion mutations have been generated (Fig. 3c). Quantification of the restriction digestion analysis of the plasmids originally containing OG:A and G:A bps recovered from E. coli harboring MutY showed a G:C base pair level of 99 ± 2 % and 40 ± 2 %, respectively, at the lesion site (Fig. 3d).
The absence of MutY leads to G:C → T:A mutations
In order to evaluate the level of repair mediated by MutY, the same experimental protocol was performed with the OG:A or G:A mismatch plasmid using an E. coli strain deficient in MutY (JM101 mutY−) (Supplementary Fig. 1). Since MutY is not present in these cells, initiation of BER cannot take place through excision of adenine and therefore G:C → T:A transversion mutations are expected to be generated upon replication of the plasmid. Upon BmtI digestion of the recovered plasmid that originally contained OG:A, a mixture of three bands was observed indicating a small amount of G:C base pair present at the lesion site (Supplementary Fig. 1a). This result is consistent with lack of repair and some faithful incorporation of C across from OG by cellular replicative polymerases. DNA sequence analysis showed that the identity of the base at the lesion site was mainly T, with a small amount of G (Supplementary Fig. 1b). Quantification of band intensities demonstrated that a G:C base pair was generated at the OG:A lesion site 29 ± 3 % of the time (Fig. 3d). This mutation frequency is similar to a previous report using a mutY− fpg−E. coli strain (BH990).27
Both the restriction fragment analysis and DNA sequencing of plasmid isolated from mutY− E. coli demonstrates that with the G:A plasmid, G:C bps are generated at a level of 33 ± 8 % (Fig. 3d and Supplementary Fig. 1). These results with OG:A and G:A provide an important baseline for the processing of these substrates in the absence of MutY activity in E. coli. Importantly, the amount of G:C bp observed with the G:A-containing plasmid is similar in the presence and absence of MutY indicating that there is not a significant amount of repair of this mismatch (Fig. 3d).
Mismatch repair alters cellular repair of G:A
Mismatch repair (MMR) participates in repairing G:A mismatches and has also been implicated to repair OG:A mismatches.28,29 MMR is initiated by the mismatch recognition activity of MutS.1 The repair assay was performed with the OG:A and G:A mismatch containing plasmids using mutY− and mutS− E. coli strains to determine the potential contribution of MMR (Supplementary Fig. 1 and Fig. 3e). The plasmid substrate in these cellular experiments is hemi-methylated and therefore contains the proper signal to recruit MMR. As expected, high levels of repair (98.1 ± 0.3 %) of the OG:A plasmid substrate were observed in the parent strain AB1157 that contains endogenous MutY and MutS. Moreover, the extent of G:C bp observed with the OG:A plasmid in the KM75 mutS− strain (98.5 ± 0.2 %) is within error of those for the parent strain AB1157, indicating that MMR is not affecting the repair of OG:A mismatches by MutY. In the GM7724 mutY− strain the generation of G:C bps is considerably reduced (42 ± 1 %) consistent with the lack of MutY to mediate the repair of the OG:A mismatch. The most significant difference observed using the MutS-deficient strain was with the G:A-containing plasmid. The extent of G:C bp in the recovered plasmid (53 ± 1 %) is close to what might be expected if both strands were replicated equally. This is in contrast to the results with the G:A plasmid recovered from all other strains (WT and mutY−) where the amount of G:C bp was near 40% (Fig. 3d,e). This suggests that MMR is acting upon the hemi-methylated G:A plasmid, where the A-strand is methylated, to preferentially degrade the G-containing strand, leading to a lower % G:C at the lesion site.
OG:Z3 base pairs are Efficiently Repaired by MutY
To assess the effect of adenine modification on the cellular repair of OG:Z3 and G:Z3 mismatches, the same strategy as described with the OG:A and G:A containing plasmids was used. The results show that when paired opposite OG, only a slight reduction in the repair of OG:Z3 to G:C bps is observed compared to OG:A bps in E. coli containing endogenous MutY (Supplementary Fig. 1). Quantification of the restriction fragment analyses showed that 92 ± 2 % of the bps at the site of the OG:Z3 bp had been converted to G:C, which is significantly greater than in the absence of MutY (33 ± 2 %) (Fig. 3d). The amount of G:C bp observed with OG:A or OG:Z3 using the strain lacking MutY are within error, indicating that Z3 replacement does not significantly affect the mutagenic properties of the base pair. With a G:Z3 mismatch, the restriction fragment analysis (Supplementary Fig. 1a) is similar to that observed for a G:A mismatch, indicating minimal generation of G:C at the lesion site above the control. The percent G:C determined for G:Z3 was 44 ± 2 % and 35 ± 10 % in the presence and absence of MutY, respectively (Fig. 3d). DNA sequencing analysis supported the results obtained by restriction fragment analysis and shows that Z3 retains coding properties of A (Supplementary Fig. 1b).
Cellular Repair of OG:FA and G:FA Mismatches
The observation that E37S MutY binds with high affinity to OG:Z3 suggested that the observed MutY-mediated repair of OG:Z3 in vivo may be due to the binding of MutY to the lesion, which recruits other repair pathways to the damaged site. To test the importance of glycosidic bond cleavage, we used the cellular repair assay with the noncleavable analogue FA opposite both OG and G (Supplementary Fig. 1 and Fig. 3d). The nucleoside analogue FA is resistant to N-glycosidic bond hydrolysis due to the electron withdrawing nature of fluorine present at the C2′ position of the sugar, which destabilizes the oxocarbenium ion transition state.30 MutY exhibits particularly high affinities for duplexes containing OG:FA bps (Kd = 0.8 ± 0.4 nM).25,31
The extents of conversion of OG:FA to G:C was significantly less than that observed with OG:A and OG:Z3 in the presence of MutY (57 ± 2 %). The G:FA mismatch was converted to G:C (44 ± 1 %) at a level that was similar to G:A and G:Z3 in the presence of MutY (Fig. 3d). In the absence of MutY, background levels of G:C were observed from both G:FA and OG:FA, which were 41 ± 5 % and 49 ± 2 %, respectively (Fig. 3d). Interestingly, the extent of G:C at the site of OG:FA is higher in the presence of MutY than in the absence, thus indicating some type of MutY-mediated repair, albeit modest, does occur. Also of note is that the amount of G:C produced at the OG:FA and G:FA bps in the absence of MutY is higher than with OG:A and G:A bps, respectively (Fig. 3d).
DISCUSSION
The activity of variants of MutY can be gauged by evaluating the ability to prevent DNA mutations in rifampicin resistance assays.25,32,33 In these experiments, the absence of MutY activity allows spontaneous mutations in the rifampicin binding site of E. coli RNA polymerase to accumulate and render rifampicin less effective as a block to transcription.14,34,35 However, such assays are not readily adaptable to evaluating the repair of a specific damaged DNA substrate or unnatural synthetic DNA nucleotides. The strategy described herein provides a simple method to introduce a specific lesion-containing mismatch plasmid into a bacterial strain of interest to evaluate BER mediated by MutY.
To evaluate the features of the OG:A base pair required for mismatch recognition and adenine removal, and how these features impact MutY-mediated repair in E. coli, we investigated two specific modifications of the OG:A substrate in detail. The importance of recognition of OG to select adenines for excision was evaluated by detailed analysis of the kinetics, binding, and in vivo repair of G:A mismatches. The Z3 analogue provided an opportunity to probe the importance of efficient catalysis of adenine removal by MutY on the overall repair of the OG:A mismatch by only altering the efficiency of base cleavage and not mismatch affinity. The various effects in vitro and in vivo of the two modifications from OG:A described herein indicate that the presence of OG is a critical requirement for efficient MutY-mediated repair.
As anticipated, this work demonstrates that an OG:A mismatch is completely repaired to G:C by endogenous MutY in E. coli.14 Complete repair at the lesion site occurs prior to replication, as no T:A base pairs are observed. In contrast, the lack of significant repair of a G:A mismatch mediated by MutY in these cellular repair assays was surprising since MutY was originally discovered as an enzyme active on G:A mismatches.6,4 The fact that the OG:A mismatch is completely repaired under the same conditions where the G:A mismatch is not significantly repaired, suggests that MutY is more proficient at locating OG:A mismatches and completing repair prior to replication. With G:A substrates, MutY is unable to discern which base within the base pair is incorrect, and therefore the repair mediated by MutY may be pro-mutagenic. Thus, the G:A mismatch may be more safely repaired by MMR that utilizes methylation at GATC to distinguish the base in the newly replicated strand from that of the parental strand. Indeed, we observe some MMR-mediated repair of G:A in our cellular assay, suggesting that MMR is more efficient at mediating cellular repair of G:A mismatches than MutY.
The kinetic data revealed that the glycosylase activity of MutY with G:A substrates is sensitive to the sequence context and is significantly reduced at physiological salt concentrations. In fact, these two factors resulted in minimal adenine removal activity by MutY from the G:A mismatch in the sequence context of the in vivo assay (duplex 1). Thus, the in vitro results are consistent with the minimal repair of the G:A mismatch in this cellular assay. Remarkably, however, the adenine glycosylase activity of MutY with OG:A bps is extremely robust, and is unaffected by these sequence contexts and buffer salt conditions. The effects of the increased NaCl concentration on the activity with G:A substrates but not OG:A substrates reveals the relative contributions of specific and nonspecific binding events in the adenine removal activity of MutY. DNA bending and distortion that is associated with forming the catalytically competent DNA-MutY intermediate is expected to be facilitated by electrostatic and hydrogen-bonding interactions of DNA backbone phosphates with amino acid side chains and backbone amides.24 In the case of G:A substrates, accessing the catalytically competent intermediate must rely heavily on nonspecific electrostatic interactions to explain the sensitivity to the increased salt concentration.36,37 In contrast, with OG:A substrates, nonspecific electrostatic interactions are not as critical for efficient adenine removal. These results underscore the importance of recognition of OG to fully engage the adenine in the active site for facile glycosidic bond cleavage. Indeed, stopped-flow fluorescence experiments with MutY and duplexes containing 2-aminopurine flanking an OG:A mismatch have suggested that recognition of OG occurs prior to extrusion of A.38 Our results indicate that MMR may be better able to recognize G:A mismatches, but does not interfere with the repair of OG:A mismatches by MutY.
The most surprising result in this work was the observation of efficient MutY-mediated in vivo repair of OG:Z3 mismatches, despite the reduced ability of MutY to catalyze Z3 removal in vitro. However, the dissociation constant measurements show that affinity for the mismatch is not significantly altered by removal of N3. This is consistent with the absence of contacts of the enzyme with N3 of A in the X-ray crystal structure of the BsMY LRC (Fig. 1).24 In addition, the removal of N3 is unlikely to affect base pairing with OG, and therefore should not globally alter the recognition features of the mismatched base pair or the ease with which MutY mediates base pair disruption. Thus, the reduced ability of MutY to remove Z3 relative to A is likely due to alterations of the electronic properties of the base. Indeed, such a modification would be expected to modulate the pKa of N7, which is likely protonated as part of the base excision process.30 Based on the positioning of a water molecule between N7 of A and Glu 43 of BsMY in the LRC X-ray structure, Glu 43 was proposed to serve as the general acid to protonate N7 of A via the water molecule as an important step in the dissociative mechanism (Fig. 1c).24 The resulting deprotonated Glu 43 was also proposed to play the role of the general base in deprotonating a second water molecule, which then is the nucleophile that attacks the oxocarbenium ion intermediate in the second step of the reaction. Thus, we hypothesize that only adenine has the proper pKa of N7 to allow for efficient protonation and deprotonation steps mediated by Glu 43.
The fact that a reduced rate for Z3 excision does not translate into a similarly reduced efficiency of repair suggests that rapid base cleavage chemistry is not required. Only large reductions in rates of base cleavage, as observed with the duplex 1 G:A substrate at physiological salt, appear to translate into reduced extents of repair. Interestingly, the extent of repair observed in the cellular assay correlates most closely with the measured dissociation constants of E37S MutY bound to the relevant mismatch-containing duplexes. E37S MutY exhibited an extremely high affinity for both OG:A and OG:Z3 mismatch-containing duplexes. With duplexes containing G:A and G:Z3 mismatches, the affinity was approximately three-orders of magnitude smaller and only slightly higher (~5-fold) than that observed with a duplex lacking a mismatch (Kd = 150 ± 60 nM).39 This suggests that the better predictor of high levels of repair is efficient binding of MutY to the mismatch. We suggest that affinity of MutY for a given mismatch may be reporting on the ability of MutY to efficiently locate the mismatch. Comprehensive studies as reported herein with other modified base pairs may help to further elaborate this correlation and provide additional support for this hypothesis.
The importance of the glycosylase function of MutY in initiating BER relative to the role of high affinity binding of MutY to a mismatch in recruiting some other type of repair pathway was also examined by evaluating the repair of plasmid substrates containing the base excision-resistant nucleotide FA opposite OG and G. The extent of repair in the presence of MutY is significantly higher for both OG:A and OG:Z3 mismatches compared to OG:FA, which supports the importance of the glycosylase function of MutY. However, the extent of G:C observed at the OG:FA site is higher in the presence of MutY than in the absence. Interestingly, it should be noted that a higher proportion of G:C base pairs is observed with both the OG:FA and G:FA mismatch-containing plasmid in the absence of MutY than with the corresponding A-containing plasmids. This increase in the control may be due to the presence of the fluorine atom in this analogue, which may affect nucleotide insertion by the DNA polymerases in vivo and alter the strand bias of DNA replication. Tight binding of MutY to the OG:FA-containing plasmid may provide an additional signal that recruits repair polymerases or other repair machinery (e.g. nucleotide excision repair) to provide the modest increase in repair (8%) afforded in the presence of MutY at the OG:FA site within the plasmid.
Taken together, this work suggests that recognition of substrates by MutY in vivo relies heavily on the identification of the OG base and less on A. In other words, modification of the adenine can be tolerated to a greater extent in vivo than perturbation of the OG lesion itself. Importantly, efficient repair requires initiation of the base excision repair pathway via the glycosylase action of MutY; however, the overall extent of repair is not directly related to the rate of base excision. The correlation with the binding affinity suggests that “finding” the mismatch is more important than rapid base-cleavage chemistry. Indeed, since repair occurs in competition with replication, efficient location of the damaged site prior to replication may become more important than rapid base cleavage. In the case of OG:A or OG:Z3, location of the mismatch by MutY stalls replication until repair is complete. Moreover, once replication of the A-strand of the OG:A bp is complete, a normal T:A base-pair is produced, and the chance for repair has been lost. This underscores that the presence of OG is the critical feature that allows for locating the mismatch prior to replication.
The importance of recognition of OG has also been highlighted by studies of inherited variants of MUTYH found in patients with MUTYH-associated polyposis.10,24 The two most common missense amino acid substitutions in MUTYH result in reduced glycosylase activity and affinity for OG-containing substrates.40 In addition, the corresponding E. coli variants are unable to distinguish OG from G.25,36 This suggests that the function of MutY and its human homologue to prevent deleterious DNA mutations is critically dependent on the ability to detect OG and select the appropriate undamaged A for excision to initiate BER.
Materials and Methods
Preparation of Oligonucleotide Substrates
DNA oligonucleotides containing the normal phosphoramidites and the nonstandard 7,8-dihydro-8-oxo-2′-deoxyguanosine (OG), 2′-deoxy-2′-fluoroadenosine (FA) and 3-deaza-2′-deoxyadenosine (Z3) phosphoramidites (Glen Research) were synthesized at University of Utah core facility. Oligonucleotides containing Z3 in the duplex 2 sequence context were synthesized as previously reported.41 Deprotection, purification, end-labeling and duplex formation was as described previously.17 Two 30 nt DNA duplex oligonucleotides were used for the in vitro experiments, duplex 1 (5′-CGATCATGGAGGCTAOCGCTCCCGTTACAG-3′• 3′-GCTAGTACCTCCGATY GCGAGGGCAATGTC-5′) and duplex 2 (5′-CGATCATGGAGCCACOAGCTCCCGTTACAG-3′ • 3′-GCTAGTACCTCGGTGYTCGAGGGCAATGTC-5′), where O = OG or G, and Y = A, Z3, or FA.
MutY Purification, Glycosylase and Binding Assays
The MutY and mutant E37S enzymes were overexpresssed and purified as described previously by our laboratory.18,19 The total concentration of protein was determined by A280 with an extinction coefficient (ε) of 77,510 M cm−1 for MutY.38 Active site concentration of MutY was determined using the approach we previously reported.17 For E37S MutY, binding titrations were first performed to determine the amount of “active” enzyme.18 Glycosylase activity assays and dissociation constants (Kd) were measured as described previously17,31,39. Buffer conditions for the glycosylase assays were 20 mM Tris-HCl pH 7.6, 10 mM EDTA, 0.1 mg/mL BSA, and 30 mM or 150 mM NaCl. The Kd reported is considered an apparent Kd since all shifted and supershifted bands are taken together in determining the concentration of “bound” enzyme. Storage phosphor autoradiography and quantification of gels was performed on a Typhoon 9400 imaging system. Data analysis was done using ImageQuaNT 5.2 and GraFit 5. In all cases, the values reported are the average of at least three separate experiments, and the error reported as the standard deviation of the sample set.
Preparation of Plasmid DNA Substrates
The pACYC177 cloning vector (New England BioLabs) was used to harbor the substrate-containing oligonucleotide duplex DNA. All plasmid DNA was purified using the Wizard Plus Miniprep kit from Promega according to the manufacturer’s protocol via a vacuum manifold. This substrate vector was prepared by double restriction digestion using BamHI and PstI of the cloning vector followed by agarose gel purification. Gel purification of DNA from agarose gels was performed using a Qiagen Gel Extraction kit using the protocol provided by the manufacturer for use with a centrifuge. This linearized vector fragment (pACYC) was combined for ligation with the oligonucleotide duplex 3 (5′-GATCCGATCATGGAGGCTAOCGCTCCCGTTACAGCTGCA-3′ • 3′-GCTAGTACCTCCGATYGCGAGGGCAATGTCG-5′). Duplex 3 contained a potential BmtI site (italics) and the appropriate overhangs for ligation into BamHI and PstI restriction sites. Duplex 3 oligonucleotides were phosphorylated and annealed as described above, except that unlabeled ATP was used. The phosphorylated substrate-containing oligonucleotide duplex 3 was ligated into the linearized pACYC vector using Quick Ligase (New England BioLabs) for 5 min at RT according to the manufacturer’s protocol. The resulting ligated substrate plasmids, pACYCOG:A, pACYCG:A, pACYCOG:Z3, pACYCG:Z3, pACYCOG:FA and pACYCG:FA, were purified via silica filter (Novagen) according to the protocol provided by the manufacturer for PCR product clean-up and stored at −20 °C.
Cell-Based Repair Assay
Each of the substrate vectors was transformed by electroporation into electrocompetent XL-1 Blue (Novagen) or JM101 (mutY::Tet) E. coli cells according to standard protocols,42 suspended in 300 μL of SOC media, and incubated at 37 °C for 1 h. An equal volume of cells (150 uL) was used to both inoculate 5 mL of LB growth media and LB agar plates containing the appropriate antibiotics, and both were incubated at 37 °C overnight. For transformations which gave >100 colonies on the LB agar plate, the LB media overnight growth was used for chloramphenicol or spectinomycin amplification of the pACYC substrate vector according to standard protocols.42 Cells were harvested by centrifugation and the pACYC substrate vector was isolated. Two aliquots (700 ng) of isolated plasmid were used for DNA sequence analysis by the DNA sequencing core facility, and for restriction fragment analysis using Bmt I (New England Biolabs). For the Bmt I digestion, the reaction used 20 units of Bmt I for a 3 h digestion at 37 °C, then an additional 20 units of Bmt I was added and the reaction incubated at 37 °C overnight. Fragments generated were analyzed by agarose gel electrophoresis using ethidium bromide (EtBr) in 1X TAE at 100 V for 1.5 hrs. Quantification of ethidium bromide (EtBr)-stained gels was performed on either a Molecular Dynamics Storm 840 or Typhoon 9400 imaging system. The completion of the BmtI digestion was assessed using a control plasmid in which a G:C-containing oligonucleotide at the lesion site was ligated into the pACYC vector and subjected to the assay as described above. The percent G:C at the lesion site was determined to be equal to [I(1748)+I(1308)]/[I(3056)+I(1748)+I(1308)]*100 where I (MW) is the intensity of the indicated molecular weight (MW) band. The fragments observed by restriction analysis were compared to the digestion completion observed with a G:C-containing plasmid at the lesion site (typically greater than 98%). The percent G:C calculated from recovered plasmids was then normalized to this control, which was adjusted to 100%. The same protocol was used for transformation and analysis of the pACYCOG:A and pACYCG:A substrate vectors in AB1157, GM7724 (mutY::Cam) and KM75 (mutS465::Tet) E. coli.
Acknowledgments
We would like to thank B. Wilcock, S. Kundu and Dr. M.A. Pope for technical assistance. We also thank Dr. Martin Marinus (UMass) for providing the AB1157, GM7724 (mutY::Cam) and KM75 (mutS465::Tet) E. coli strains. Drs. Jeff Miller and Mark Michaels (UCLA) provided the pKKYEco plasmid, and JM101 (mutY-) E. coli strain. This work was supported by National Institutes of Health grants to S.S.D. (CA67985) and E. T. K. (GM072705) and National Institutes of Health pre-doctoral traineeships to A.L.L. (GM08537) and V.L.O. (GM08537 and CA093247). The DNA sequencing facility at the University of Utah Medical School is supported in part by an NIH-NCI grant (5P30CA43014).
Footnotes
Author Contributions
A.L.L., V.L.O, and S.S.D. designed the experiments. A.L.L and V.L.O performed the experiments. T.K. synthesized the Z3-containing duplex 2 oligonucleotide. S.S.D and E.T.K supervised all experiments. A.L.L, V.L.O and S.S.D wrote the manuscript. E.T.K. read the manuscript and made comments on the manuscript.
References
- 1.Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA Mismatch Repair: Functions and Mechanisms. Chem Rev. 2006;106:302–323. doi: 10.1021/cr0404794. [DOI] [PubMed] [Google Scholar]
- 2.Su SS, Lahue RS, Au KG, Modrich P. Mispair Specificity of Methyl-directed DNA Mismatch Correction. J Biol Chem. 1988;263:6829–6835. [PubMed] [Google Scholar]
- 3.Lu AL, Chang DY. Repair of Single Base-Pair Transversion Mismatches of Escherichia coli in Vitro: Correction of Certain A/G Mismatches is Independent of dam methylation and Host mut HLS gene Functions. Genetics. 1988;118:593–600. doi: 10.1093/genetics/118.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radicella JP, Clark EA, Fox MS. Some mismatch repair activities in Escherichia coli. Proc Natl Acad Sci USA. 1988;85:9674–9678. doi: 10.1073/pnas.85.24.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngheim Y, Cabrera M, Cupples CG, Miller JH. The mutY gene: A mutator locus in Escherichia coli that generates G:C to T:A transversions. Proc Natl Acad Sci USA. 1988;85:2709–2713. doi: 10.1073/pnas.85.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Au KG, Cabrera M, Miller JH, Modrich P. Escherichia coli mutY gene product is required for specific AG to CG mismatch correction. Proc Natl Acad Sci USA. 1988;85:9163–9166. doi: 10.1073/pnas.85.23.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Au KG, Clark S, Miller JH, Modrich P. Escherichia coli mutY gene encodes an adenine glycosylase active on G-A mispairs. Proc Natl Acad Sci USA. 1989;86:8877–8881. doi: 10.1073/pnas.86.22.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu AL, Chang DY. A Novel Nucleotide Excision Repair for the Conversion of an A/G Mismatch to C/G Base Pair in E. coli. Cell. 1988;54:805–812. doi: 10.1016/s0092-8674(88)91109-9. [DOI] [PubMed] [Google Scholar]
- 9.David SS, Williams SD. Chemistry of Glycosylases and Endonucleases Involved In Base-Excision Repair. Chem Rev. 1998;98:1221–1261. doi: 10.1021/cr980321h. [DOI] [PubMed] [Google Scholar]
- 10.David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sung JS, Demple B. Roles of base excision repair subpathways in correcting oxidized abasic sites in DNA. FEBS J. 2006;273:1620–1629. doi: 10.1111/j.1742-4658.2006.05192.x. [DOI] [PubMed] [Google Scholar]
- 12.Michaels ML, Miller JH. The GO system Protects Organisms from the Mutagenic Effect of the Spontaneous Lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) J Bact. 1992;174:6321–6325. doi: 10.1128/jb.174.20.6321-6325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaels ML, Tchou J, Grollman AP, Miller JH. A Repair System for 8-oxo-7,8-dihydrodeoxyguanosine. Biochemistry. 1992;31:10964–10968. doi: 10.1021/bi00160a004. [DOI] [PubMed] [Google Scholar]
- 14.Michaels ML, Cruz C, Grollman AP, Miller JH. Evidence that MutY and MutM combine to prevent mutations by an oxidatively damaged form of guanine in DNA. Proc Natl Acad Sci USA. 1992;89:7022–7025. doi: 10.1073/pnas.89.15.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Tassan N, et al. Inherited variants of MYH associated with somatic G:C to T:A mutations in colorectal tumors. Nature Gen. 2002;30:227–232. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- 16.Sampson JR, Jones S, Dolwani S, Cheadle JP. MutYH (MYH) and colorectal cancer. Biochem Soc Trans. 2005;33:679–683. doi: 10.1042/BST0330679. [DOI] [PubMed] [Google Scholar]
- 17.Porello SL, Leyes AE, David SS. Single-Turnover and Pre-Steady-State Kinetics of the Reaction of the Adenine Glycosylase MutY with Mismatch-Containing DNA Substrates. Biochemistry. 1998;37:14756–14764. doi: 10.1021/bi981594+. [DOI] [PubMed] [Google Scholar]
- 18.Francis AW, Helquist SA, Kool ET, David SS. Probing the Requirements for Recognition and Catalysis in Fpg and MutY with Nonpolar Adenine Isosteres. J Am Chem Soc. 2003;125:16235–16242. doi: 10.1021/ja0374426. [DOI] [PubMed] [Google Scholar]
- 19.Chmiel NH, Golinelli MP, Francis AW, David SS. Efficient Recognition of substrates and substrate analogs by the adenine glycosylase MutY requires the C-terminal domain. Nucleic Acids Res. 2001;29:553–564. doi: 10.1093/nar/29.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCann JAB, Berti PJ. Adenine Release is Fast in MutY-catalyzed Hydrolysis of G:A and 8-oxo-G:A DNA Mismatches. J Biol Chem. 2003;278:29587–29592. doi: 10.1074/jbc.M212474200. [DOI] [PubMed] [Google Scholar]
- 21.Pope MA, Porello SL, David SS. E. coli AP endonucleases enhance the turnover of the adenine glycosylase MutY with G:A substrates. J Biol Chem. 2002:22605–22615. doi: 10.1074/jbc.M203037200. [DOI] [PubMed] [Google Scholar]
- 22.Pope MA, David SS. DNA damage recognition and repair by the murine MutY homologue. DNA Repair. 2005;4:91–102. doi: 10.1016/j.dnarep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Hang B, Singer B. Protein-Protein Interactions Involving DNA Glycosylases. Chem Res Toxic. 2003;16:1181–1195. doi: 10.1021/tx030020p. [DOI] [PubMed] [Google Scholar]
- 24.Fromme JC, Banerjee A, Huang SJ, Verdine GL. Structural basis for removal of adenine mispaired with 8-oxoguanine by MutY adenine DNA glycosylase. Nature. 2004;427:652–656. doi: 10.1038/nature02306. [DOI] [PubMed] [Google Scholar]
- 25.Chmiel NH, Livingston AL, David SS. Insight into the Functional Consequences of Inherited Variants of the hMYH Adenine Glycosylase Associated with Colorectal Cancer: Complementation Assays with hMYH Variants and Pre-steady-state kinetics of the Corresponding Mutated E. coli Enzymes. J Mol Biol. 2003;327:431–443. doi: 10.1016/s0022-2836(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 26.Pearson CG, Shikazono N, Thacker J, O’Neill PO. Enhanced mutageneic potential of 8-oxo-7,8-dihydroguanine when present within a cluster DNA damage site. Nucleic Acids Res. 2004;32:263–270. doi: 10.1093/nar/gkh150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shikazono N, Pearson C, O’Neill PO, Thacker J. The roles of specific glycosylases in determining the mutagenic consequences of clustered DNA base damage. Nuc Acids Res. 2006;34:3730–3738. doi: 10.1093/nar/gkl503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Winkler ME. Reduction of the GC to TA Transversion Mutation by Overexpressino of MutS in E. coli K-12. J Bact. 2000;182:5025–5028. doi: 10.1128/jb.182.17.5025-5028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai H, Lu AL. Physical and Functional Interactions between Escherichia coli MutY Glycosylase and Mismatch Repair Protein MutS. J Bact. 2007;189:902–909. doi: 10.1128/JB.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berti PJ, McCann JAB. Toward a Detailed Understanding of Base Excision Repair Enzymes: Transition State and Mechanistic Analyses of N-Glycoside Hydrolysis and N-Glycoside Transfer. Chem Rev. 2006;106:506–555. doi: 10.1021/cr040461t. [DOI] [PubMed] [Google Scholar]
- 31.Chepanoske CL, Porello SP, Fujiwara T, Sugiyama H, David SS. Investigation of Substrate Recognition by E. Coli MutY using Substrate Analogs. Nucleic Acids Res. 1999;27:3197–3204. doi: 10.1093/nar/27.15.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golinelli MP, Chmiel NH, David SS. Site-Directed Mutagenesis of the Cysteine Ligands to the [4Fe-4S] Cluster of Escherichia coli MutY. Biochemistry. 1999;38:6997–7007. doi: 10.1021/bi982300n. [DOI] [PubMed] [Google Scholar]
- 33.Bai H, et al. Functional characterization of two human MutY homolog (hMYH) missense mutations (R227W and V232F) that lie within the putative hMSH6 binding domain and are associated with hMYH polyposis. Nucleic Acids Res. 2005;33:597–604. doi: 10.1093/nar/gki209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller JH. A Short Course in Bacterial Genetics. Cold Spring Harbor; NY: 1992. [Google Scholar]
- 35.Wehrli W, Knusel F, Schmid K, Staehlin M. Interaction of Rifamycin with Bacterial RNA polymerase. Proc Natl Acad Sci USA. 1968;61:667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livingston AL, Kundu S, Henderson-Pozzi M, Anderson DW, David SS. Insight into the Roles of Tyrosine 82 and Glycine 253 in the Escherichia coli Adenine Glycosylase MutY. Biochemistry. 2005;44:14179–14190. doi: 10.1021/bi050976u. [DOI] [PubMed] [Google Scholar]
- 37.Record MT, Ha JH, Fisher MA. Analysis of equilibrium and kinetic measurements to determine thermodynamic origins of stability and specificty and mechanism of formation of site-specific complexes between proteins and helical DNA. Methods Enzymol. 1991;208:291–343. doi: 10.1016/0076-6879(91)08018-d. [DOI] [PubMed] [Google Scholar]
- 38.Bernards AS, Miller JK, Bao KK, Wong I. Flipping Duplex DNA Inside Out: A double base-flipping reaction mechanism by Escherichia coli MutY Adenine Glycosylase. J Biol Chem. 2002;277:20960–20964. doi: 10.1074/jbc.C200181200. [DOI] [PubMed] [Google Scholar]
- 39.Porello SL, Williams SD, Kuhn H, Michaels ML, David SS. Specific Recognition of Substrate Analogs by the DNA Mismatch Repair Enzyme MutY. J Am Chem Soc. 1996;118:10684–10692. [Google Scholar]
- 40.Pope MA, Chmiel NH, David SS. Insight into the Functional Consequences of hMYH variants associated with colorectal cancer: distinct differences in the adenine glycosylase activity and the response to AP endonuclease of Y150C and G365D murine MYH. DNA Repair. 2005;4:315–325. doi: 10.1016/j.dnarep.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Maki AS, Kim T, Kool ET. Direct Comparisons of A-strand and T-strand Minor Groove Interactions in DNA Curvature at A tracts. Biochemistry. 2004;43:1102–1110. doi: 10.1021/bi035340m. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Press; Cold Spring Harbor: 1989. [Google Scholar]