Abstract
During the last decade, a number of new developments have emerged in the field of capillary electrochromatography (CEC). This paper focuses only on monolithic columns prepared from synthetic polymers. Monolithic columns have become a well-established format of stationary phases for CEC immediately after their inception in the mid 1990s. They are readily prepared in situ from liquid precursors. Also, the control over both porous properties and surface chemistries is easy to achieve. These advantages make the monolithic separation media an attractive alternative to capillary columns packed with particulate materials. Since the number of papers concerned with just this single topic of polymer-based monolithic CEC columns is large, this overview describes only those approaches this author found interesting.
Keywords: Monolith, Capillary, Electrochromatography, Microfluidic devices, Review
1 Prologue
It is the second half of the 1990s. The monoliths are in their infancy with a multiplicity of their formats being developed just within the last few years [1–4]. Only a handful of groups were working with these new materials and exploring their applications mostly for the separations in liquid chromatography. At the same time, electrochromatography rose from ashes like the mythical Phoenix. This separation method has been proposed by Pretorius already in 1974 [5]. He realized the advantage of the flat flow profile generated by electroosmotic flow (EOF) that was supposed to reduce peak broadening thus affording better column efficiency in both thin layer and column chromatography. However, for a couple of decades his technique has been almost forgotten. The 1990s featured search for new microseparation methods with vastly enhanced efficiencies, peak capacities, and selectivities needed for the emerging fields of life science and pharmaceuticals and CEC became one of the targets. The new interest in CEC has also been fueled by discoveries such as that by Smith who demonstrated that extreme column efficiencies exceeding one million of plates could be achieved using CEC in packed capillary columns although these were difficult to reproduce [6]. Results like these evoke curiosity of many researchers and jump started CEC.
Capillary electrochromatography has been defined as a high-performance liquid phase separation technique carried out in columns packed with media containing ionizable functionalities that utilizes flow driven by electroosmosis and enables to achieve significantly improved performance compared to high performance liquid chromatography (HPLC). The frequently published definitions that call CEC a hybrid of capillary electrophoresis (CE) and HPLC are actually not completely correct. In fact, electroosmotic flow is not the major feature of CE and HPLC packings do not need to be ionizable to work well. The electric field applied across the capillary also affects the partitioning of solutes and their retention, features unknown in CE and HPLC alone [7,8].
Although capillary columns packed with typical bonded silica beads have been known for many years [9], they were not finding too many applications before the mid 1990s due to a variety of reasons including difficulty with detection of tiny amounts of separated analytes and lack of commercial equipment suitable for these microseparations. However, the rebirth of CEC changed the map completely since by definition CEC is run in capillaries. Thus, column technologies became the force driving the development of CEC.
Since most of the “eletrochromatographers” begun their carrier in the field of liquid chromatography, the legacy of HPLC on the development of columns for CEC is rather obvious. For example, HPLC-like “hardware”, such as frits and packed columns, were originally widely used. The solvent slurry packing appeared to be very popular technique that has been transferred directly from the HPLC. However, packing efficient capillaries for CEC is more complex than that used for packing of the standard analytical size columns. Thus, a number of various multistep packing technologies have to be designed that enabled packing particles into narrow bore capillary columns [10,11]. The list of typical individual steps required to fabricate an efficient CEC column was long and included the following [12]:
Attaching an in-line end-frit and packing the column by pumping slurry of beads and solvent into the capillary under high pressure upon sonication
Flushing the packed column with water at high pressure to replace the solvent
Preparing the outlet end-frit at the desired distance from the column end by sintering the silica beads using heating to a temperature of over 550°C
Removing the in-line end-frit and flushing out the extra-column packing materials using reversed flow direction
Sintering of the packing materials to create the inlet end-frit at a distance representing the desired packed segment length followed by the removal of the polyimide coating from the detection window close to the outlet frit
Cutting off the excess capillary close to the inlet frit
Equilibrating the packed capillary with the desired mobile phase
This packing procedure is not trivial and requires specific skills to afford highly efficient capillaries reproducibly. The major challenge of the packing procedure appeared to be the in situ fabrication of retention frits. Even after these hurdles were handled successfully, the charged particles themselves tended to move in the electrical field during the CEC process and the columns performance changed during the application.
Difficulties with packed columns have led to search for other options. The most obvious was not to use any packing and relay on open tubular formats [13]. For example, Pesek studied the use of open capillaries in order to avoid tedious packing procedures. Since solute-stationary phase interactions are key to the CEC process, appropriate moieties must be bound to the capillary wall. However, the wall surface available for modification is severely limited. Therefore, Pesek used chemical etching to increase the surface area and created features ranging “from spikes of silica material extending 3–5 µm from the surface, to a series of hills or sand dunes, to large uniform boulder-like pieces of silica on the surface” and functionalized them using his silanization/hydrosilylation process to attach the alkyl moieties [14]. Another approach reported by Horváth applied open tubular format with a layer of reactive polymer attached to the wall prepared via in situ polymerization followed by functionalization [15]. Despite good separations achieved with the open tubular column formats, their sample loading capacity has always been small and detection difficult.
As said in the first paragraph of this introduction, the rise of CEC happened in the time when monolithic separation media just began enjoying popularity within the chromatographic community. Therefore, it did not take long for monoliths to find their way in CEC. And they did it the big way since they promised to easily solve most of the problems impairing the other column technologies described above.
Most of the “monolithic” approaches can be categorized in two groups. The first one sticks with the columns tediously packed with beads followed by their fixation to form a monolithic structure. For example, Dittmann at al. developed a simple method including packing of a capillary with octadecylsilica (ODS) beads followed by drawing a heated wire along the capillary to achieve sintering of the beads [16]. Horváth et al. sintered the contents of a capillary column packed with ODS beads by heating the entire column to 360°C in the presence of a sodium bicarbonate solution [17]. Another set of techniques “immobilized” the packed particles by formation of a sol-gel structure filling the interstitial volumes [18–21]. All these methods helped to solve the problem of column stability since the fused/immobilized beads cannot move but did not avoid the fabrication of frits, one of the critical operations in the preparation of CEC columns.
The second approach to monolithic CEC columns relayed on their preparation in situ from low molecular weight precursors. Here again, several different approaches have been designed and demonstrated. Interestingly, very little has been published prior to the year 2000 on use of silica-based capillary columns in CEC [22–24]. In contrast, many papers appeared during the late 1990s that described the use of polymeric monoliths prepared from acrylamide type monomers, styrenics, and methacrylates. In fact, three seminal papers covering these columns originating from Hjertén’s, Novotny’s, and our group were published in 1997 [25–27]. All the monolithic materials used in these studies were prepared using redox or thermally initiated free radical polymerizations. Polymer-based monolith then became very popular and a number of publications dealing with this column technology rapidly followed.
As a result of their unique properties, monolithic materials have attracted considerable attention. Perhaps the most appealing aspect of the monolithic polymers is their ease of preparation. The simple polymerization process performed directly within the confines of a capillary avoids all the problems related to both frit formation and packing. Also, columns of virtually any length and shape are easily accessible. The polymerization mixture may also be prepared using a wide variety of monomers, allowing a nearly unlimited choice of both matrix and surface chemistries. This flexibility enables the easy tailoring of both the interactions that are required for specific separation modes and the level of EOF generated by the support. Finally, the control that can be exerted over the polymerization process itself enables the facile optimization of the porous properties of the monolith that directly affect the flow rate and chromatographic efficiency of the system.
Interestingly, the second half of the 1990s is also known as the time during which the microfluidic separation devices emerged [28,29]. While their use for the separations in HPLC technique were less successful [30] since the efficient packing of chips was difficult to achieved, CEC was deemed to be much better suited for the implementation on chip. One of the major advantages of electrochromatography compared to HPLC is the ease with which it can be miniaturized to a microfluidic format. Since the fluid flow is driven by EOF, the simplest separation device does not need to include mechanical pumps or valves [31]. In addition, the flat flow profile generated by EOF minimizes dispersion of analyte bands during their passage through the stationary phase and allows very high plate counts to be achieved in a short length “column”. Since EOF is largely independent of channel or particle size, monolithic stationary phases with small pores can be used, thus facilitating solute mass transfer without generating the large pressure drop associated with classical pressure driven HPLC. Thus, electrochromatography on chip seemed to be an attractive choice for “lab-on-a-chip” separations due to its excellent separation performance and relatively easy implementation. The potential and the early developments of chip electrochromatography was soon summarized in a few very good review articles [32–34].
In our original report published in Electrophoresis in 2000 [35] that triggered request for this paper we wrote: “Obviously, this simple procedure (producing monoliths) works well with capillaries that have easily adjustable length. However, the position of the channel on a chip-based device is fixed and the separation medium must therefore be located only within the assigned space. Since adjustments in the length and position of the monolith are difficult to achieve after polymerization, it is best to ensure that the monolith is only prepared at the required location within the channel. This precise positioning would be difficult to achieve using a thermally initiated polymerization. In contrast, a light initiated polymerization process is very well suited to achieve monolith formation within a specified space. Using a mask, the polymerization may be restricted to the irradiated areas while monomers do not convert to polymer in those areas that are not irradiated. The same technology has been widely used for microelectronic patterning.“ We also stated: “Although our target is a microchip containing a bed of monolithic separation medium prepared by in situ UV initiated polymerization, the more readily available capillary columns are a good substitute for micromachined chips and are currently much less costly. Therefore, we carried out our study using the capillary format and CEC separation as a model for the microchip separation devices“ [35].
Now, ten years later, many things changed. First, validity of our assumption that monoliths in standard capillaries are a good substitute for non-circular shaped conduits has been experimentally confirmed [36]. Both photoinitiated polymerization of monoliths in capillaries as well as in microfluidic channels and their modification via photografting are relatively common. The following lines are briefly accounting for the major development in monolithic porous polymer CEC columns and related microfluidic technologies that occurred since our original manuscript has been written for this journal. To narrow the scope, most of the attention is paid to recent reports. It needs to be emphasized that this paper is not a comprehensive review since a number of excellent review articles summarizing in great detail various aspects of CEC using monolithic columns have been published recently [37–52]. Also, the readers should understand that selection of all of the bits and pieces presented below is subjective and biased by what I thought to be interesting or curious.
2 Poly(styrene-co-divinylbenzene) monoliths
Although polystyrene-based monoliths are quite popular in HPLC applications [53,54], their incidence in CEC remains limited and only narrow extent of work have been carried out after the pioneering work of Horváth’s group [55]. Less favorable reactivity ratios characterizing copolymerization of hydrophobic aromatic monomers with polar ionizable monomers that have to be included to generate EOF appear to be one of the reasons for restricted interest in this chemistry.
Since thermally initiated polymerizations require long reaction times and UV initiated photopolymerization is not well suited for the preparation of monoliths from UV absorbing aromatic monomers, Zhang et al. used 15 min long microwave irradiation in a standard home appliance to prepare poly(styrene-co-divinylbenzene-co-methacrylic acid) monolith in a capillary [56]. This monolithic column was then tested in standard and pressurized CEC and low pressure LC. Unfortunately, neither the extent of conversion of monomers to polymer nor pore volume of these monoliths were characterized to confirm that 15 min reaction time is long enough for complete transition of all monomers to polymer. The best column efficiency achieved for non-retained thiourea was mere 18,000 plates/m. This value is significantly smaller than those found for other monoliths. Thus, this approach remains for the time being a curiosity rather than a viable technique for the preparation of well performing monolithic CEC columns.
Methacrylic acid is a weak acid and generates EOF only after being ionized at higher pH (e.g [56–58]). In contrast, strong acid monomers such as those containing sulfonic acid functionalities drive EOF even at low pH values. Haung at al. copolymerized styrene, divinylbenzene, and vinylsulfonic acid and studied effects of process variables such as composition of the polymerization mixture and reaction time [59]. They measured conversion of the monomers to polymer and found that it reached 56.5% after a reaction time of 18 h and did not increase significantly thereafter. The authors do not provide any explanation for this observation. It can be hypothesized though that vinylsulfonic acid does not copolymerize well with styrene and divinylbenzene and remains dissolved in the porogens after the polymerization of other monomers is completed. A simple determination of the sulfonic acid functionalities in the monolith might shed some light on this problem. However, more curious is the fact that extension in the reaction time led to monoliths with larger pore sizes. For example, a pore size of 4.8 µm has been found after 6 h while monolith prepared by 18 h long polymerization featured pores with a size of 7.8 µm. Again, no explanation for this counterintuitive observation is provided. These columns separated organic acids and the best column efficiency was only 44,000 plates/m. In order to improve the efficiency, Huang used in the following study styrenesulfonic acid as the EOF generating monomer [60]. This time, the reaction rate was much faster as a result of better tuned monomer reactivity ratios and a complete conversion was achieved after 24 h. Also the performance of these columns improved significantly and column efficiencies of over 180,000 plates/m were recorded.
The hydrophobicity of poly(styrene-co-divinylbenzene) monoliths may not be sufficient to retain and separate less hydrophobic compounds. Therefore, several approaches have been tested to increase the overall hydrophobicity of these monoliths. For example, Huang et al. prepared monoliths in PEEK, fused silica, or stainless steel tubing and then alkylated the pore surface with 1-chlorooctadecane using Friedel-Crafts reaction [61]. This process led to monolithic column with enhanced hydrophobicity that enabled very good separation of peptides using HPLC.
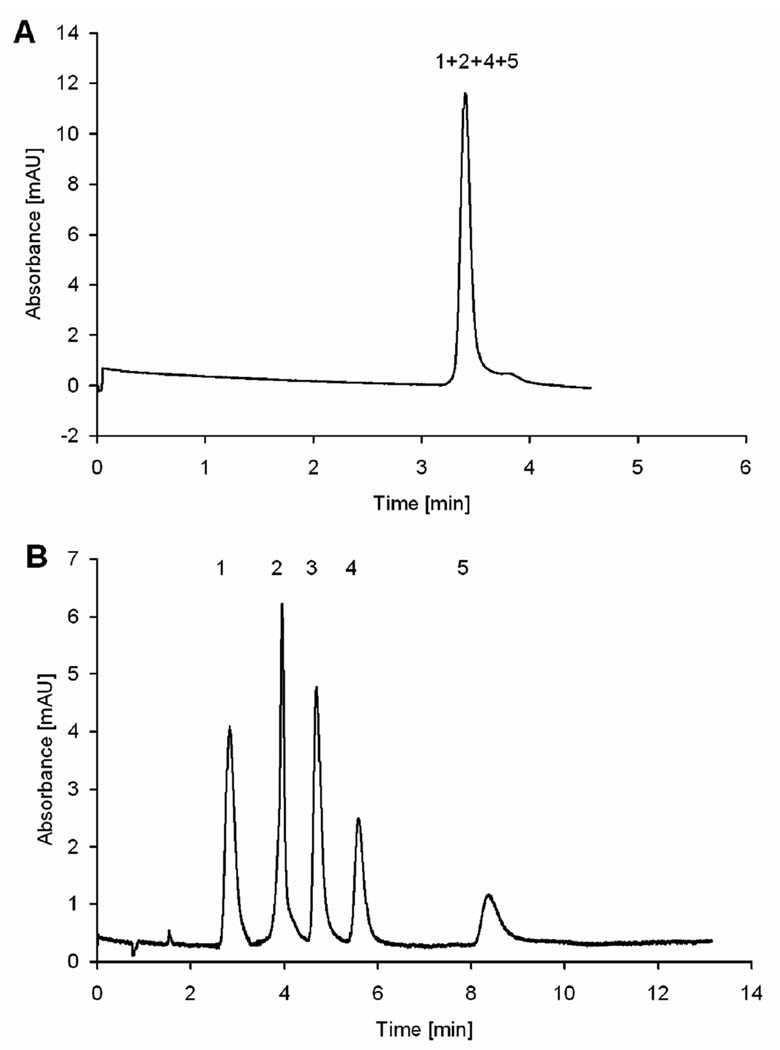
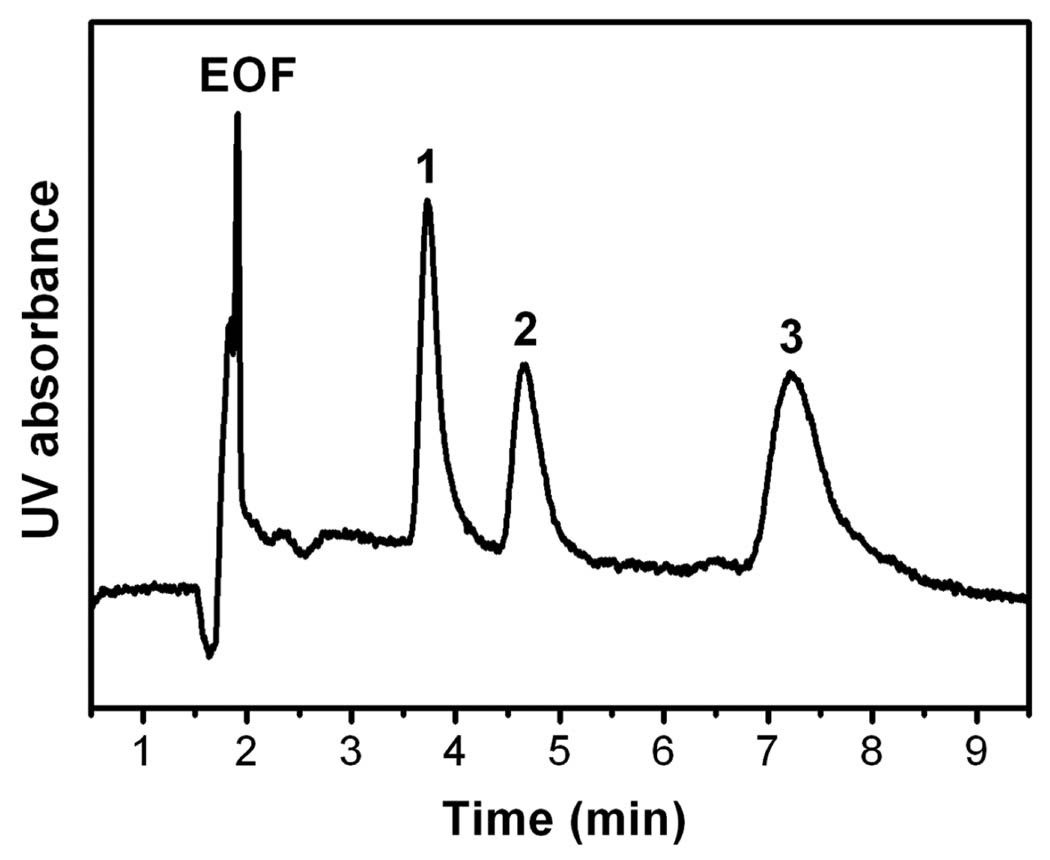
Jandera’s group extended this approach to the preparation of monolithic capillaries applicable to both HPLC and CEC [62]. They also compared performance of 1-chlorooctadecane alkylated monolith with its counterpart prepared via thermally initiated “grafting to” process using solutions of stearyl methacrylate and azobisisobutyronitrile (ABIN).Free radicals formed in the solution within pores initiated polymerization of the methacrylate monomer and the attachment of propagating chains to the wall was achieved through the residual double bonds originating from divinylbenzene. In contrast to HPLC conditions with pressurized flow, the column efficiencies in CEC did not depend on flow velocity. Yet, the origin nof EOF remains unclear since no ionizable functionalities are present in the monolith. However, it is likely that adsorbed ions are responsible for charging the surface [63,64]. Fig. 1 shows that while the unmodified poly(styrene-co-divinylbenzene) monolith did not separate phenolic acids under CEC conditions, excellent separation has been achieved with the alkylated column.
Fig. 1.
Electrochromatographic separation of phenolic acids using monolithic poly(styrene-co-divinylbenzene) column (A) unmodified column and (B) alkylated with 1-chlorooctadecane (Reprinted with permission from ref. [62]. Copyright 2007 Wiley-VCH). Mobile phase: 5 mmol/L phosphate buffer, pH 2.5; applied voltage, 20 kV; electrokinetic injection, 3 s, −5 kV, detection: UV (214 nm). Peaks: (1) thiourea, (2) gallic acid, (3) vanillic acid, (4) syringic acid, and (5) ferulic acid (0.1 mg/mL each in buffer).
3 Polyacrylamide copolymers
Hjertén’s group was the first to report the preparation of CEC columns based on acrylamide copolymers dissolved in aqueous solutions [65]. The original approach was complex, requiring a multiple steps [25]. In order to simplify the tedious preparation method, they then developed a simpler procedure [66]. The same group later described another method for the preparation of a acrylamide and piperazine diacrylamide-based monolithic capillary column that was used for CEC separation of proteins in a gradient of the mobile phase [67]. Hoegger and Freitag modified the Hjertén’s procedure and prepared a variety of monolithic acrylamide-based CEC columns in the presence of ammonium sulfate as a pore size modulating component [68]. Their approach allowed them to adjust both rigidity and porous properties of the monoliths and to achieve excellent separations.
The use of aqueous polymerization solution certainly exhibits some aspects of the “green” chemistry but makes very difficult to include hydrophobic monomers that are needed to achieve interactions desired for the reversed phase CEC separations. While Hjertén circumvent this problem by using a surfactant [25,66], Al-Rimawi and Pyel used hydroxypropyl-β-cyclodextrins as the solubilizing agent that enabled to prepare homogenous solutions including different proportions of piperazine diacrylamide, vinylsulfonic acid and alternatively isobornyl, adamantyl, cyclohexyl, or phenyl methacrylate [69]. As could be expected, an increase in the percentage of the methacrylate monomer led to enhanced hydrophobicity and retention of alkylphenones and other analytes, and afforded decent column efficiencies of up to 120,000 plates/m. They assumed that the solubilizing agent slips out of the monomer during polymerization [70] and is removed from the column during the 2 h long washing with water. However, no evidence to this end was presented.
In continuation of these efforts, Wahl et al. prepared a pseudorotaxane complex of hydrophobic crosslinker N,N’-ethylenedianilinediacrylamide with methylated β-cyclodextrin. Its structure has been confirmed spectroscopically. This complex crosslinker was then mixed in phosphate buffer with piperazine diacrylamide, N-isopropylacrylamide, and vinylsulfonic acid, and copolymerized. In contrast to monovinyl monomers used previously, cyclodextrin cannot slip off the complex with divinyl crosslinker since both its ends are incorporated in the polymer chains and remain part of the monolith. Indeed, NMR study confirms presence of cyclodextrin in the polymer. The optimized monolith enabled excellent separations of aromatic compounds such as substituted benzaldehydes with a column efficiency of up to 260,000 plates/m.
Despite the undeniable success, the use of purely aqueous-based polymerization systems for the preparation of monolithic capillaries for CEC also has some limitations and additional means discussed above are required to dissolve hydrophobic monomers. This increases the complexity of the polymerization mixture. In contrast to the "fixed" solubilizing properties of water, the wealth of organic solvents possessing polarities ranging from highly non-polar to extremely polar enables the formulation of mixtures with solvating capabilities that may be tailored over a very broad range. An additional feature of organic solvents is their intrinsic ability to control the porous properties of the monoliths.
Novotny’s group simplified the incorporation of highly hydrophobic ligands into acrylamide-based matrices by using mixtures of aqueous buffer and organic solvent N-methylformamide to prepare homogeneous polymerization solutions of acrylamide, methylenebisacrylamide, acrylic acid, and C4, C6, or C12 alkyl acrylate [26]. Columns with high efficiencies for carbohydrates were only obtained when the polymerization was performed in the presence of poly(ethylene oxide) co-porogen dissolved in the polymerization mixture. The range of potential analytes was later extended to steroids [71], and bile acids [72]. Dong et al. modified this protocol and prepared monolithic CEC columns from a solution of acrylamide, methylenebisacrylamide, and AMPS in dimethylsulfoxide/dodecanol. In contrast to redox initiation typical of acrylamide chemistry, the polymerization was initiated thermally using ABIN as the initiator. Note that no poly(ethylene oxide) has been added. Yet, the desired porous structure with sufficiently large pores could be created from optimized mixture. This column was then used for the efficient separation of various basic compounds, alkaloids, drugs, and peptides with column efficiencies reaching up to 200,000 plates/m.
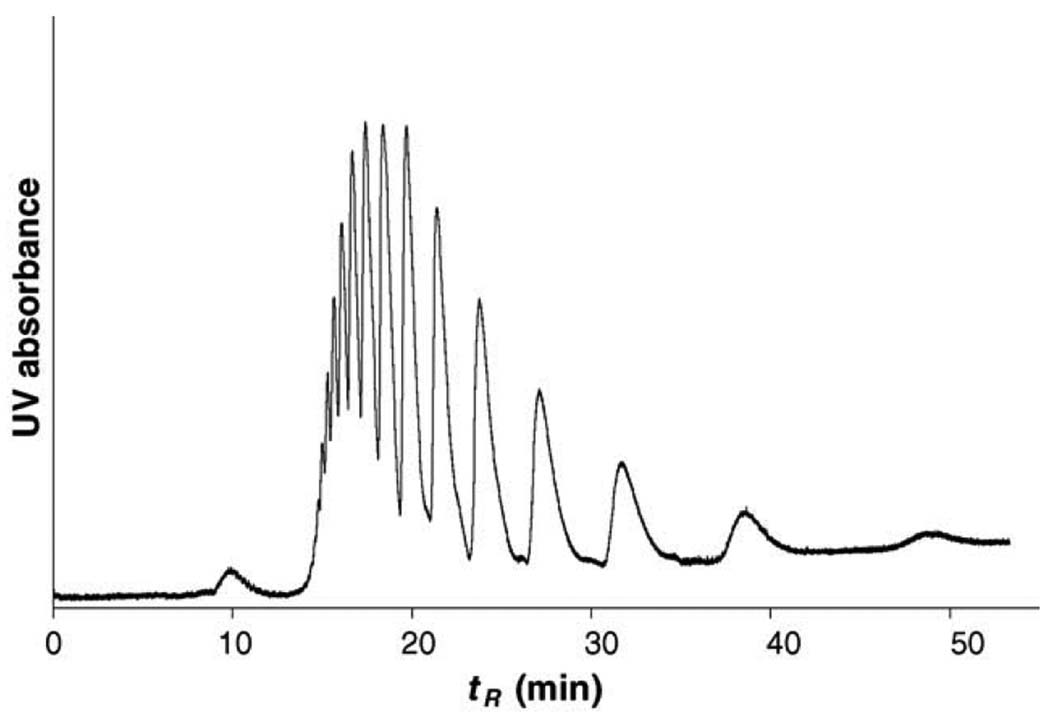
Vast majority of the CEC separation has been carried out in the reversed phase mode. However, this separation technique may not be suitable for hydrophilic compounds. This is why separation in hydrophilic interaction liquid chromatography (HILIC) are recently gaining on popularity [73]. Although HILIC has been assumed operative in several CEC separations (e.g. [69]), Novotny’s group is the most prominent in studying separations in this mode [74–76]. They demonstrated the power of this technique with CEC separations of saccharides [77]. Fig. 2 shows effect of percentage of acetonitrile (ACN) on the model separation of maltooligosaccharides labeled with 2-aminobenzamide to facilitate detection. The enhanced retention at the higher ACN percentages confirms the HILIC mechanism. Using an optimized hydrophilic monolithic column comprising acrylamide, N-[tris(hydroxymethyl)methyl]acrylamide, piperazine diacrylamide, and vinylsulfonic acid, very high column efficiencies of up to 350,000 plates/m have been found for glucopyranan oligomers. Interestingly, they observed a rapid deterioration of column performance when methylenebisacrylamide was used as a crosslinker. The column efficiency decreased by 40% after 45 runs while only a 10% decrease was monitored for the piperazine diacrylamide crosslinked monolith.
Fig. 2.
Effect of percentage of acetonitrile in the mobile phase on separation of linear oligosaccharides. (Reprinted with permission from ref. [77]. Copyright 2007 Elsevier) Column: Poly(acrylamide-co-methylenebisacrylamide) monolith, total length 35.5 cm, active length 27 cm; Analytes: maltooligosaccharides (Glc4–Glc10) tagged with 2-aminobenzamide (ca. 0.3 mg/mL), injection 2 kV/10 s; Mobile phase: acetonitrile/ 240 mmol/L ammoniumformate/water; laser induced fluorescence detection at ~600 V/cm.
This group also developed an interface enabling eluent from monolithic CEC column to be deposited on a MALDI-MS plate [76]. While CEC-ESI-MS interface has been designed and developed at the early times of CEC [78–82], this was the first example of successful combination of CEC with MALDI. They used their own poly(acrylamide-co-cyanoethyl acrylate-co-vinylsulfonic acid-co-methylene bisacrylamide) monolithic capillary column run in HILIC mode for the CEC separation of oligosaccharide ladder and glycans released from bile-salt stimulated lipase.
4 Polymethacrylate copolymers
Acrylates and methacrylates represent a very rich family of monomers enabling access to numerous functionalities residing in the ester part of the molecule that are easily copolymerized with a variety of crosslinkers such as ethylene dimethacrylate, butanediol diacrylate, and trimethylolpropane trimethacrylate to afford both the desired well-controlled porous structure and pore surface chemistry. Interestingly, glycidyl methacrylate leads the list of monomers used for the preparation of monolithic capillary columns for CEC [83–88]. We have introduced this monomer in the preparation of porous polymer beads already in 1975 [89], and the first monoliths consisted of this monomer as well [2,3]. The other highly prioritized monomer is butyl methacrylate [90–92] that we also pioneered in our seminal papers [93–95]. We have also developed a unique porogenic system comprising water, 1,4-butanediol, and 1-propanol that dissolved hydrophobic alkyl methacrylate monomer, ethylene dimethacrylate crosslinker, highly hydrophilic AMPS, and AIBN initiator and enabled to obtain the porous structure providing for good CEC separations. This mixture also remains quite popular in the current literature [85,92,96–100]. Another often used porogenic system consists of cyclohexanol and dodecanol [83,84,88,96,101] that we first used in 1975 [89]. Needless to say that many clones of these mixtures as well as some completely new porogenic systems have also been successfully used for the preparation of monolithic CEC columns (vide infra).
Thermally initiated polymerization mechanism is a “classic” since it was the first approach used to prepare monolithic columns in general [3] and those for CEC in particular [93] and remains the most often used approach to monoliths. For example, the Amsterdam group examined the practical aspects of using monolithic columns prepared from butyl methacrylate, ethylene dimethacrylate, and 2-(methacryloyloxy)-ethyltrimethyl ammonium chloride (META) [90]. They varied composition of the porogens as well as percentage of monomers in the polymerization mixture and found that the column efficiency was strongly analyte dependent. An interesting application is demonstrated with the separation of differently PEGylated components of a technical surfactant Triton-X100 (Fig. 3). In order to avoid a significant decrease in efficiency for more retained analytes, they proposed running the separations at higher temperature and using tetrahydrofuran instead of ACN as the strong solvent [96]. They also compared this CEC column with both packed and silica monolith columns using Poppe plots [102]. The high pore volume methacrylate monolith afforded the best performance.
Fig. 3.
CEC separation of Triton-X using a poly(butyl methacrylate-co-ethylene dimethacrylate-co-2-(methacryloyloxy)ethyltrimethyl ammonium chloride) monolithic column with a modal pore size of 600 nm. (Reprinted with permission from ref. [90]. Copyright 2007 Elsevier). Column active length 25 cm; Mobile phase 35:65% (v/v) acetonitrile-tris buffer (pH 8.0, 5mmol/L); UV detection at 210 nm.
El Rassi’s group is very active in the preparation of monolithic CEC columns from a variety of methacrylate monomers and their use for the separation of biopolymers. For example, they were the first to use pentaerythritol diacrylate monostearate as the crosslinker [103] and stearyl methacrylate as the hydrophobic monomer [104]. These excellent monolithic columns enabled very rapid separation of proteins [105]. The poly(stearyl methacrylate-co-pentaerythritol diacrylate monostearate) monolithic column was used to demonstrate 2-dimensional separation of proteins using a combination of capillary isoelectric focusing followed by CEC [106]. They also prepared monolithic poly(glycidyl methacrylate-co-ethylene dimethacrylate) column, immobilized lectin through the epoxide groups, and used it for the CEC separation of glycoproteins in lectin affinity mode [83]. Their recent monolithic column consisting of glycidyl methacrylate or 2,3-dihydroxypropyl methacrylate crosslinked with ethylene dimethacrylate or trimethylolpropane trimethacrylate appeared to be well suited for the separation of polar compounds including glycans [88].
An unusual approach to monolithic columns for the high efficiency separation of opiates was developed by Lin et al. [87]. They first prepared a poly(glycidyl methacrylate-co-ethylene dimethacrylate-co-3-sulfopropyl methacrylate) column, washed away the porogens, and then filled the column with 1 mol/L hydrochloride acid to quench the epoxide groups. This reaction is likely to afford diol functionalities via a simple hydrolysis as well as chlorohydrin groups formed through the reaction of epoxide with HCl. The same group also copolymerized iso-butyl methacrylate, ethylene dimethacrylate, and N,N-dimethylethylallylamine in the presence of dimethylformamide-1,4-butanediol porogen [107]. This column then separated a variety of mixtures of neutral and ionized compounds.
While thermally initiated polymerization is common, redox initiation of methacrylate monomers is in contrast to acrylamides much less used. The reason can simply be the onset of the polymerization reaction immediately after admixing the activator, typically N,N,N’,N’-tetramethylethylenediamine to the solution of peroxodisulfate initiator in monomer/porogen mixture. Therefore, this mixture must be used immediately and cannot be stored. Interestingly, the redox initiation has been used first for the preparation of LC columns [108] then later extended to CEC and used for the preparation of monolithic capillaries from butyl methacrylate, ethylene dimethacrylate, and META [109].
Our publication that appeared in 2000 [35] launched an interest in the preparation of monolithic capillary columns using UV light to initiate the polymerization process [101,110–114]. The most important condition for success is that the polymerizing system must be transparent for UV light. This condition disqualifies monomers that adsorb light in the UV range and common capillaries coated with non-transparent polyimide. In contrast, acrylates and methacrylates as well as Teflon coated capillaries are well suited for use with this initiation mechanism.
For example, Augustin et al. studied effect of polymerization conditions on porous properties and chromatographic performance of monoliths formed from butyl or hexyl acrylate, butanediol diacrylate, AMPS, and methacryloyloxypropyltrimethoxy silane, which was added as adhesion promoter [110]. Addition of the adhesion promoter in the polymerization mixture is a creative way to achieve the strong covalent attachment of the monolith to capillary wall. They found that an open tubular column was formed at a high dose, while a very reproducible monolithic CEC columns affording 300,000 plates/m were obtained under optimal conditions.
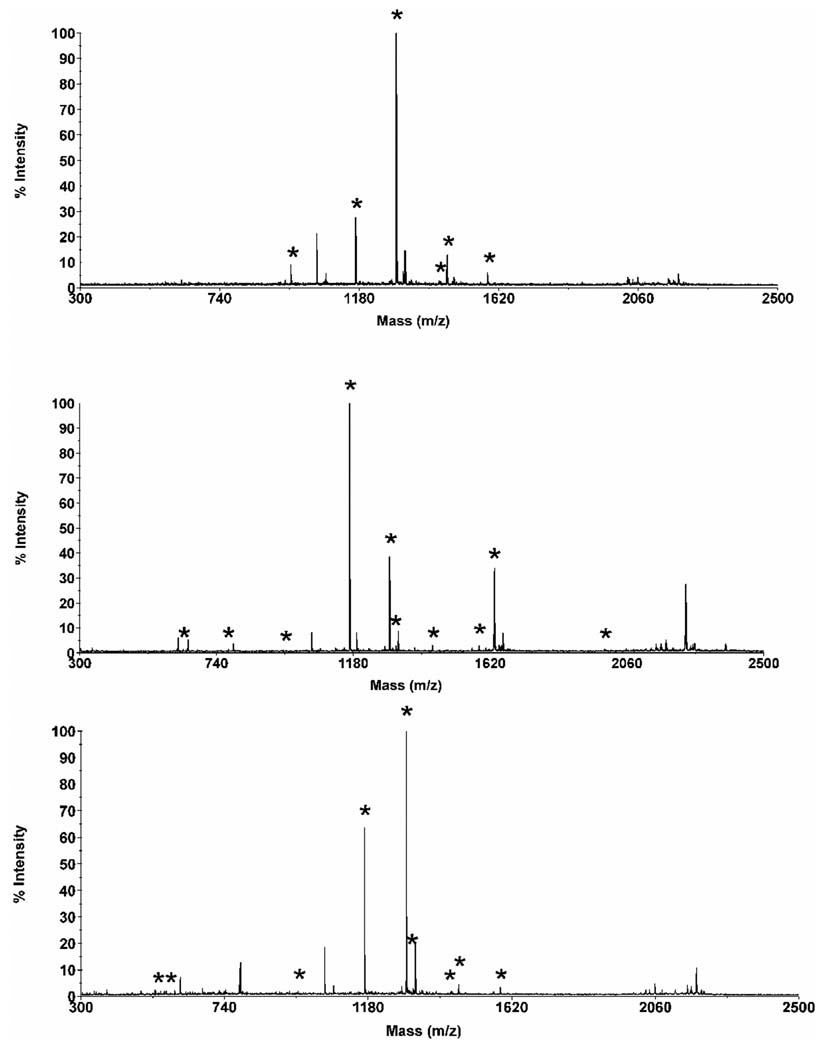
A similar polyacrylate-based monolithic column this time bearing cationic functionalities was prepared via photopolymerization of a mixture of hexyl acrylate, butanediol diacrylate, 2-(acryloyloxy)ethyltrimethylammonium chloride, azobisisobutyronitrile (photoinitiator), acetonitrile, phosphate buffer, and ethanol [115]. The polymerization process was initiated with UV light at 360 nm. These columns performed well in the separations of alkylbenzenes, substituted anilines, basic drugs, peptides, and a protein digest under optimized conditions. The separations were affected by both interaction of the peptides with the stationary phase and their own electrophoretic mobility. To exemplify the separation power of these columns, we separated a mixture of ten well-defined peptides and a tryptic digest of cytochrome c. In the latter, the fractions of eluent containing peptides of the digest separated in the monolithic column were collected and characterized using MALDI mass spectrometry. The result is shown in Fig. 4
Fig. 4.
Electrochromatogram of the separation of a tryptic digest of cytochrome c using a long monolithic capillary column followed by collection of three fractions of eluent (A) and MALDITOF mass spectra of peptides contained in the fractions. (Reprinted with permission from ref. [115]. Copyright 2008 Wiley-VCH) Conditions: Poly(hexyl methacrylate-co-1,3-butenediol dimethacrylate-co-2-(acryloyloxy)ethyltrimethyl ammonium chloride) monolithic column, total length 72 cm, active length 64.5 cm, 100 µm i.d. Mobile phase: 30:70 v/v acetonitrile −40 mmol/L Tris phosphate buffer pH 2.5; Hydrodynamic injection 0.3 MPa, 90 s. Voltage: −30 kV. Detection at 200 nm. Asterisk marks peaks that could be assigned to a specific peptide
An example of a less typical approach is the preparation of a capillary CEC column via only 12 min long irradiation of a mixture of N-acryloylsuccinimide, ethylene dimethacrylate, toluene, and ABIN with 365 nm UV light [113]. Despite the use of reaction mixture containing 65% toluene, which is an aromatic solvent not transparent for the used wavelength, the SEM micrographs indicate well developed monolithic structure. The advantage of using N-acryloylsuccinimide as the reactive monomer is twofold: It facilitates reaction with C5 – C7 alkylamines to afford hydrophobic moieties needed for separation and, simultaneously, creates carboxylic acid functionalities that drive EOF [113,114]. Although the column efficiencies for small molecules were not very high, this column separated proteins in mobile phase comprising ACN and borate buffer pH 10 as shown in Fig. 5.
Fig. 5.
Electrochromatographic separations of a mixture of three proteins on hexylaminederivatized monolithic N-acryloylsuccinimide-based column (Reprinted with permission from ref. [113]. Copyright 2007 Wiley-VCH). (1) myoglobin, (2) albumin, (3) a-lactalbumin. CEC conditions: mobile phase: acetonitrile–borate buffer (5 mmol/L, pH 10) 50–50 (v/v). Columns length: 21 cm to detector, 31.2 cm overall. Injections 10 kV for 10 s, running voltage 30 kV at 258C, detection at 214 nm.
5 Grafted surface chemistries
Surface grafting is a very versatile tool enabling preparation of a vast variety of surface chemistries from a single “generic” monolith [116,117]. For example, the pore surface within a “classical” poly(butyl methacrylate-co-ethylene dimethacrylate) monolithic column were grafted with META and AMPS [101]. The grafting time was found to have a pronounced effect on the magnitude of EOF and also retention of the column. In order to control both EOF and hydrophobicity, we also co-grafted META and butyl acrylate.
Most of previously reported separations of proteins by CEC using monolithic capillary columns required the use of a rather acidic separation buffer in order to ensure that all the proteins carried sufficient positive charge and do not interact with the similarly charged stationary phase.
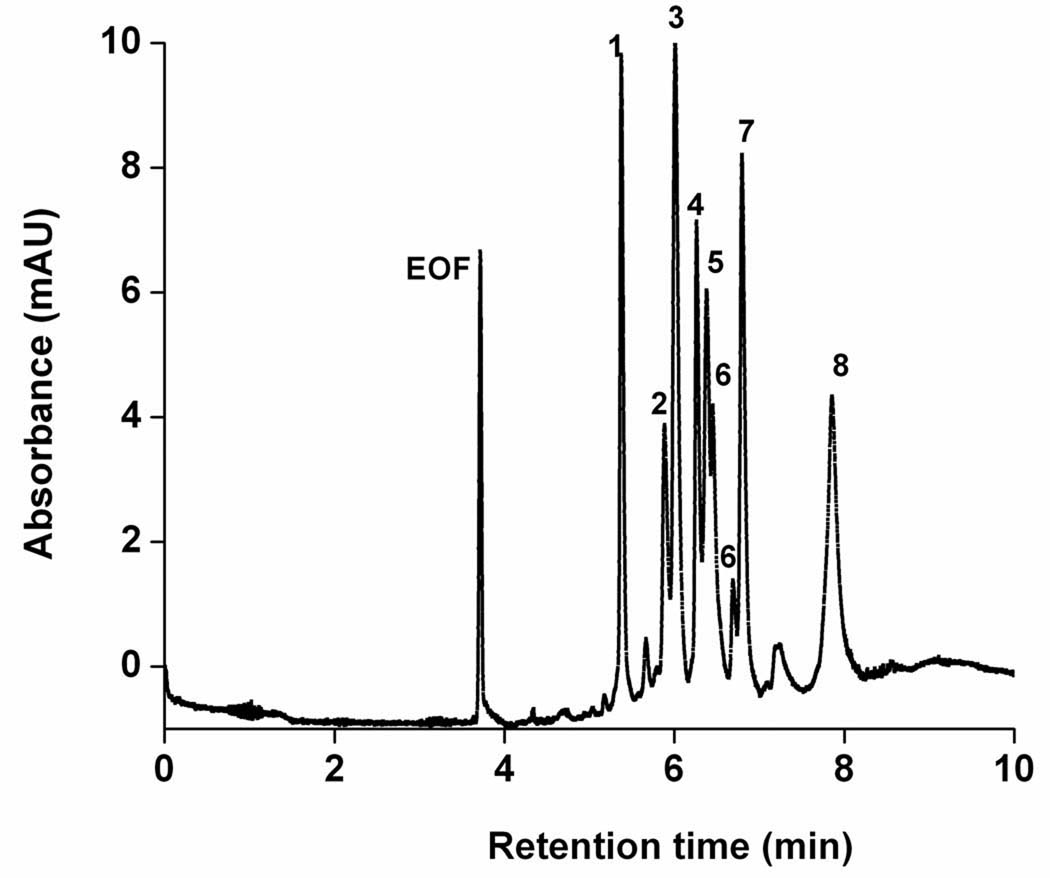
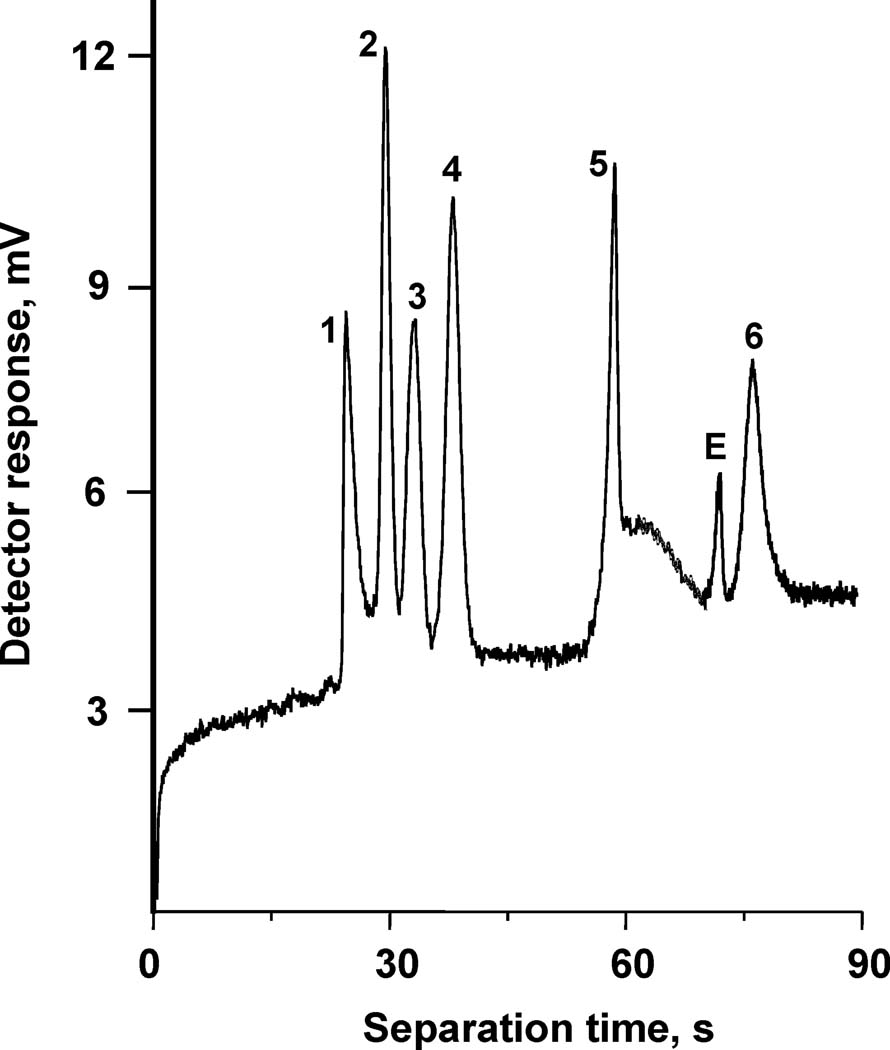
We also used photografting to create a novel stationary phase for capillary electrochromatography using photoinitiated grafting of two layers of polymer chains onto the pore surface of a porous polymer monolith in capillary [118]. In order to achieve the desired retention, the original monolith with optimized porous properties was grafted with an “interior” layer consisting of the ionizable monomer, 2-acrylamido-2-methyl-1-propanesulfonic acid, followed by a “covering” layer of hydrophobic poly(butyl acrylate) chains. This technique afforded monolithic CEC columns that facilitate electroosmotic flow while preventing ionized analytes from undesired interacting with the charged surface functionalities at neutral pH. Interestingly, grafting of the second layer did not adversely affect the EOF. We achieved a full shielding of the large peptides and proteins from the ionizable functionalities while achieving an excellent chromatographic performance. This approach allowed for the first time the independent optimization of both electroosmotic flow and retention properties in CEC columns. This stationary phase enabled very efficient isocratic separations of mixtures of peptides, including some that are highly basic and would be affected by unshielded charges. An example of this separation is shown in Fig.6
Fig. 6.
Separation of a mixture of basic and acidic peptides using a monolithic capillary column with layered chemistries. (Reprinted with permission from ref. [118]. Copyright 2004 American Chemical Society). Column: BuMA/EDMA, 8.5 cm×50 µm i.d., pore size 1.6 µm; mobile phase: 20 mmol/L ammonium acetate in 1:1 water-acetonitrile; 30 kV, 25 °C; injection 5 kV for 3 s. Peaks: substance P (1), [Arg8]-vasopressin (2), bradykinin potentiator B (3), bradykinin fragment 1–5 (4), oxytocin (5), Gly-Tyr (6), EOF (E).
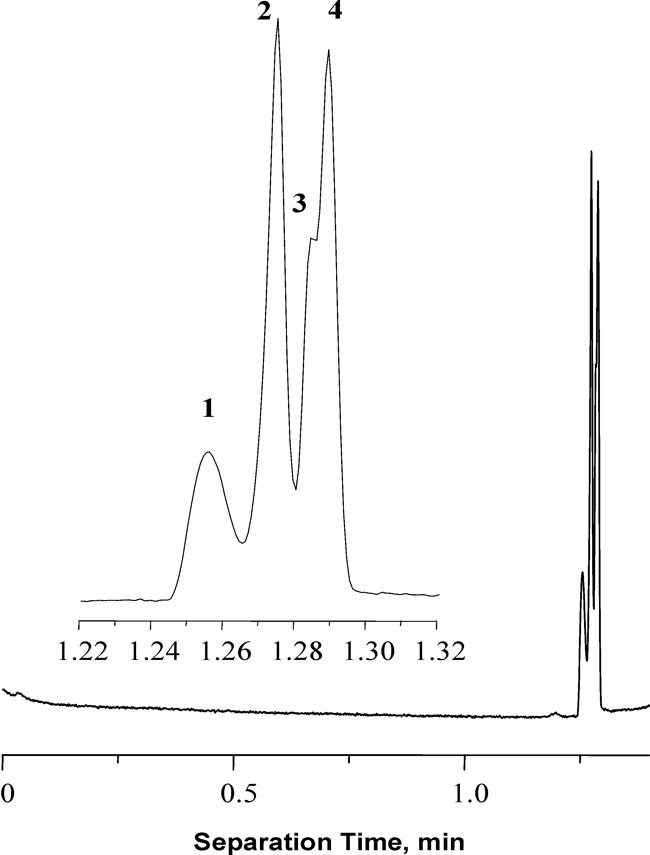
Using grafting, we also have prepared monolithic columns allowing for the extremely rapid and efficient separation of proteins at neutral pH [119]. A less common monolithic column with zwitterionic functionality was produced by photoinitiated grafting of poly(N,N-dimethyl-N-(2-methacryloyloxyethyl)-N-(3-sulfopropyl) ammonium betaine). We found that the highly reproducible separation of four proteins could be achieved in less than 1.3 min. with column efficiencies of up to 4 million plates/m as shown in Fig. 7. In contrast to the Smith’s observations [6], these extremely high efficiencies were perfectly repeatable and suggested that a narrow migration zone was maintained, which was likely due to a focusing mechanism
Fig. 7.
Separation of proteins using monolithic capillary grafted with N,N-dimethyl-N-(2-methacryloyloxyethyl)-N-(3-sulfopropyl) ammonium betaine. (Reprinted with permission from ref. [119]. Copyright 2004 Elsevier) Conditions: 50 µm capillary column total length 34.5 cm, monolith 8.5cm, 1 min grafting; mobile phase 5 mmol/L phosphate buffer (pH 7.0)/acetonitrile (20+80); voltage −15 kV; overpressure in both vials 0.8 MPa; temperature 60 °C. Peaks (1) cytochrome c, (2) holotransferrin, (3) ribonuclease a, (4) myoglobin.
6 Monoliths with a gradient of chemistry
Elution using a gradient of mobile phase is common in the chromatographic separations of large molecules such as proteins, nucleic acids, and synthetic polymers. This technique has also very early been demonstrated in CEC although home-made systems had to be used since commercial instruments enabling gradient CEC were and still are not available [120–123]. In addition to the gradient of the mobile phase, separations in gradients of temperature [124] and voltage [125] have also been demonstrated. However, until recently nobody used the gradient of column chemistry.
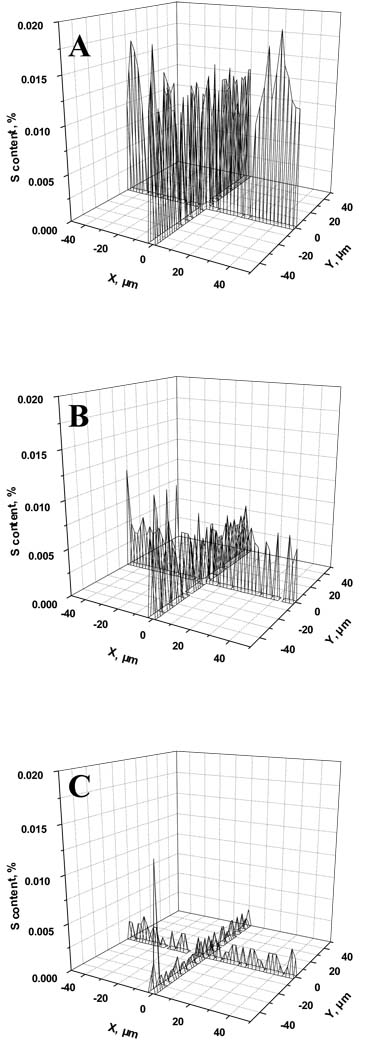
Our group used the photoinitiated grafting to create a continuous gradient of poly(2-acrylamido-2-methyl-1-propanesulfonic acid) grafts in pores of a poly(butyl methacrylate-co-ethylene dimethacrylate) monolith [126]. The process is simple and takes advantage from effect of the exposure time on the extent of grafting. Drawing a shutter along the capillary exposes the monolith to UV light in a gradient of time thus creating the desired gradient of chemistry. Alternatively, filter with a gradient of density can also be used. Fig. 8 visualizes the content of sulfur at different locations of the column and demonstrates presence of the desired gradient. This column exhibited an increase in retention compared to its non-gradient counterpart and a significantly increased efficiency for the separation of salicylic and aminosalicylic acids.
Fig. 8.
Sulfur content determined using electron probe microanalysis for monolith grafted with a gradient of 2-acrylamido-2-methyl-1-propanesulfonic acid using the neutral density filter. Irradiation through the neutral density filter with decreasing transmission from step 1 (A) to step 5 (B) and step 9 (C). (Reprinted with permission from ref. [126]. Copyright 2004 Wiley-VCH)
Recently, Maruska et al. reported the preparation of CEC column with a stepwise gradient of hydrophobicity [127]. They prepared a series of polymerization mixtures combining piperazine diacrylamide, N-(hydroxymethyl)acrylamide, diallyldimethyl ammonium chloride with increasing amounts of the hydrophobic components - (3-allylamino-2-hydroxypropyl)dodecyldimethylammonium chloride and hexyl acrylate. These polymerization mixtures were then sequentially filled in the capillary and polymerized to afford the stepwise gradient. Compared to a homogeneous monolith, they found twice as high column efficiency for the separation of alkyl benzoates when the gradient column was used. This result confirms our early observations.
7 Monoliths coated with nanoparticles
While most of the methods controlling the surface chemistry of porous polymer monoliths described so far relies on copolymerization of functional monomers, chemical modification of reactive groups of the monolith, or grafted chains formed from functional monomers, a new technique has been recently introduced to the monolithic arena –functionalization with nanoparticles [128–131]. Its roots stem from an old technique used by Dionex Corporation for the preparation of column packings for ion chromatography. Haddad’s group set the stage for the use of this technique in CEC [132]. They modified silica based monolith with 70 nm latex particles bearing quaternary ammonium groups and used the column for the separation of inorganic ions.
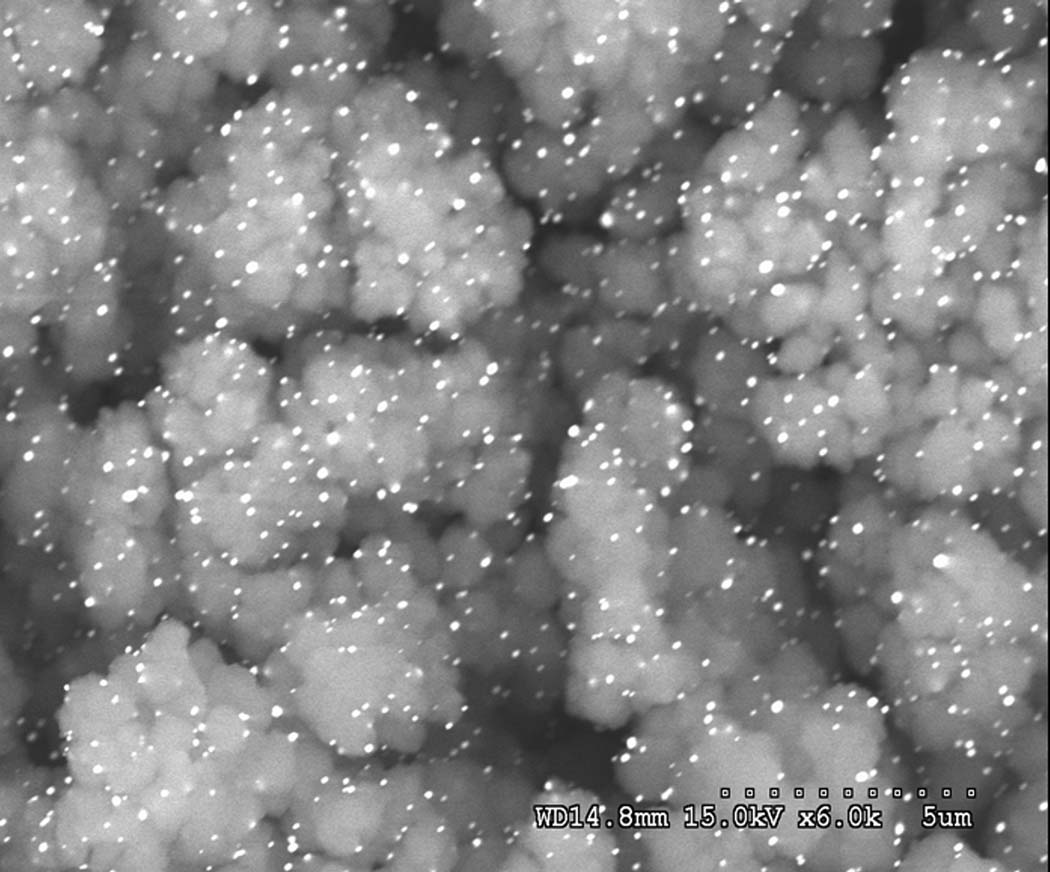
Gold nanoparticles represent another type of nanoparticles useful in CEC [133]. So far, these particles have only been used to coat inner walls of fused silica capillaries [134–137]. We recently extended the use of gold nanoparticles to functionalization of pore surface in porous polymer monoliths. The procedure is simple: First, poly(glycidyl methacrylate-co-ethylene dimethacrylate) monolith reacts with cysteamine to afford thiol functionalities and then the nanoparticles are formed in situ via reaction of chloroauric acid with trisodium citrate. Fig. 9 shows SEM of monolithic structure containing gold nanoparticles. They contribute significantly to the separation performance. While no separation of peptides could be observed using the poly(glycidyl methacrylate-co-ethylene dimethacrylate) monolith and only a poor separation is achieved after the modification with cysteamine, a good separation of peptides was obtained with the column containing the gold nanoparticles.
Fig. 9.
SEM micrograph of the modified poly(glycidyl methacrylate-co-ethylene dimethacrylate) monolith containing 15.4 atom.% gold nanoparticles.
8 Open tubular monolithic formats
As mentioned in Introduction, open-tubular formats were intensively studied from the very beginning of modern CEC [13,138]. While some groups just modified the silica walls inside the capillary [139–141], Horváth’s group was the first to coat the capillary with a monolithic polymer layer consisting of poly(chloromethylstyrene-co-divinylbenzene) [15]. This polymer was then functionalized with dimethyldodecylamine to create both ionizable and hydrophobic functionalities and the column was used for the CEC separation of proteins. Years later, Huang et al. got inspired by this early work and prepared porous layer open tubular (PLOT) CEC columns from a mixture of 2-hydroxyethyl methacrylate, 4,4-dimethyl-1-vinylazlactone (VAZ), dodecanol, and AIBN [142]. Bovine serum albumin was then immobilized on this polymer and the column used for the CEC separation of four amino acids. Although it remains a mystery why the porous structure is formed in the absence of a crosslinker, which typically prevents collapse of the structure, and what drives the EOF, the scanning electron micrograph (SEM) pictures indicate porosity.
We used our two-step approach to functionalized monoliths [116] to create highly efficient PLOT CEC columns. The preparation consisted in photoinitiated polymerization affording a generic poly(butyl methacrylate-co-ethylene dimethacrylate) monolith followed by photografting of ionizable monomer [2-(methacryloyloxy)ethyl]-trimethylammonium chloride [143]. This monolithic CEC column enabled very fast separations while exhibiting an efficiency of up to 400,000 plates/m. Fig. 10 shows a rapid separation of benzene derivatives achieved using pressurized flow and higher temperature.
Fig. 10.
Separation of benzene derivatives on coated 20-µm I.D. column with a film thickness of 0.5 µm operated at 60°C, applying 50 mbar inlet pressure and an applied voltage of 30 kV. Chromatographic conditions: 40:60% ACN:tris buffer (10 mmol/L, pH = 8). UV detection at 200 nm. Insert: SEM micrograph of the cross section of 50-µm I.D. PLOTcapillary columns prepared in revolving capillary at 100 rpm. Polymerization conditions: 50:50% butyl methacrylate-ethylene dimethacrylate solution in 1-octanol, 0.1 wt% 2,2-dimethoxy-2-phenylacetophenone (with respect to monomers). UV irradiation at 254 nm for 10 min at 30°C. Insert shows SEM micrograph of the PLOT capillary.
Obviously, monolithic PLOT columns represent a promising approach with applications reaching beyond the borders of CEC. For example, Karger’s group used these columns for very efficient HPLC separations of proteins and peptides in long, narrow bore 10 µm I.D. capillaries [144–146].
9 Wide bore columns
Most of the modern CEC work has been carried out in capillaries with an internal diameter of up to 100 µm. Interestingly, the pioneering work in CEC featured tubes with a much larger diameters such as 1 mm claimed by Pretorius [5]. The smaller diameters were always preferred since they facilitate dissipation of the Joule heat. However, large columns are desirable for high efficiency “preparative” separations. For example, we used a 550 µm I.D. capillary column with monolithic frits to separate bacitracin and taxol already in 2000 [147]. Yuan et al. [148] recently published a report demonstrating electrochromatography with a monolithic column prepared from acrylamide, methylenebisacrylamide, butyl acrylate and acrylic acid using Palm and Novotny’s recipe [26] in a 1.2 mm I.D. quartz tube. Quartz is known to exhibit high heat conductivity thus enabling to keep the temperature in the 10 cm long column under control. Despite the large size of the column, the efficiency remains within a reasonable limits with a plate height of 22 µm. A quite high sample capacity of 3 µg proves the expectation that larger samples can be separated without observing any significant decrease in the column efficiency. The performance of this “mega” CEC column was demonstrated with the separation of food dyes.
10 Concluding remarks
Fig. 11 shows that number of publications grew quite significantly from the days of its rebirth in the mid 1990s. This graph and the above text clearly document that the reports of CEC death are greatly exaggerated. Sure, electrochromatography is less frequently used than both its older cousins - liquid chromatography and electrophoresis. Some scientists claim that this is due to reduced reproducibility of this technique, limited knowledge of processes controlling the separation, lack of dedicated instrumentation, etc. These complaints might include a grain of truth. However, many reports clearly demonstrate that an excellent injection-to-injection, day-to-day, and batch-to-batch reproducibility can be achieved [11,90]. Obviously, to do so, a careful work practices are required. The theoretical base of CEC has also been established with seminal work of Horváth [55] and several other authors contributed thereafter. For example, Talarek’s group published an extensive study of effects of electrohydrodynamics in hierarchically structured monolithic columns focusing on the concentration polarization as the key phenomenon in CEC [149].
Fig. 11.
Annual frequency of publications concerned with CEC (Source SciFinder).
My feeling is that the major issue is the lack of applications in which the established separation methods fail. One positive example is the separation of β and γ positional isomers of tocopherol that is very difficult using HPLC. In contrast, separation of tocopherols has been readily achieved using CEC [97,150]. Similarly, CEC may have a great potential in separations requiring high resolution power such separation of enantiomers for which the separation factor α is close to 1 [151] or separation of complex protein and peptide mixtures in order to significantly increase the peak capacity [104]. I can also see a range of applications in 2-dimensional systems where CEC would represent one dimension. Reports demonstrating this application are already emerging [106]. Microfluidics is a quite broad field where CEC is useful as well [152–155] although its full potential has not been tapped yet.
Since many groups all around the world continue practicing CEC in its numerous incarnations, some of which have been described above, I don’t believe that CEC may disappear from the radar of the separation science anytime soon. It is likely that there will be eventually a bad need for a method like this, which will brought it to the level of the currently well-established techniques. Capillary electrophoresis is a good example that this can happen.
Acknowledgements
Work at the Molecular Foundry was supported by the Director, Office of Science, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. Support by grants of the National Institute of General Medical Sciences (GM048364) as well as the National Institute of Biomedical Imaging and Bioengineering (EB006133), National Institutes of Health is gratefully acknowledged.
Abbreviations
- ABIN
azobisisobutyronitrile
- ACN
acetonitrile
- AMPS
2- acrylamido-2-methyl-1-propanesulfonic acid
- CEC
capillary electrochromatography
- EOF
electroosmotic flow
- HILIC
hydrophilic interaction liquid chromatography
- ODS
octadecylsilica
- PLOT
porous layer open tubular
- VAZ
4,4-dimethyl-1-vinylazlactone
- META
2-(methacryloyloxy)ethyltrimethyl ammonium chloride
References
- 1.Hjertén S, Liao JL, Zhang R. J. Chromatogr. 1989;473:273–275. [Google Scholar]
- 2.Tennikova TB, Svec F, Belenkii BG. J. Liquid Chromatogr. 1990;13:63–70. [Google Scholar]
- 3.Svec F, Frechet JMJ. Anal. Chem. 1992;54:820–822. [Google Scholar]
- 4.Minakuchi H, Nakanishi K, Soga N, Ishizuka N, et al. Anal. Chem. 1996;68:3498–3501. doi: 10.1021/ac960281m. [DOI] [PubMed] [Google Scholar]
- 5.Pretorius V, Hopkins BJ, Schieke JD. J. Chromatogr. 1974;99:23–30. [Google Scholar]
- 6.Smith NW, Evans MB. Chromatographia. 1995;41:197–203. [Google Scholar]
- 7.Grimes BA, Liapis AI. J. Coll. Interface Sci. 2001;234:223–243. doi: 10.1006/jcis.2000.7269. [DOI] [PubMed] [Google Scholar]
- 8.Grimes BA, Liapis AI. J. Chromatogr. A. 2001;919:157–179. doi: 10.1016/s0021-9673(01)00789-0. [DOI] [PubMed] [Google Scholar]
- 9.Novotny M, Ishii D. Microcolumn separations: Columns, instrumentation, and ancillary techniques. Elsevier: Amsterdam; 1985. [Google Scholar]
- 10.Svec F. Adv. Biochem. Eng. Biotechnol. 2002;76:1–47. doi: 10.1007/3-540-45345-8_1. [DOI] [PubMed] [Google Scholar]
- 11.Deyl Z, Svec F, editors. Capillary Electrochromatography. Elsevier: Amsterdam; 2001. [Google Scholar]
- 12.Saevels J, Wuyts M, Schepdael AV, Roets E, et al. J. Pharmaceut. Biomed. Anal. 1999;20:513–520. doi: 10.1016/s0731-7085(99)00053-9. [DOI] [PubMed] [Google Scholar]
- 13.Jinno K, Sawada H. Trends Anal. Chem. 2000;19:664–675. [Google Scholar]
- 14.Pesek JJ, Matyska MT. J. Chromatogr. 2000;887:31–41. doi: 10.1016/s0021-9673(00)00180-1. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Zhang J, Horvath C. J. Chromatogr. 1999;858:91–101. doi: 10.1016/s0021-9673(99)00795-5. [DOI] [PubMed] [Google Scholar]
- 16.Dittmann MM, Rozing GP, Ross G, Adam T, et al. J. Cap. Electrophoresis. 1997;5:201–212. [PubMed] [Google Scholar]
- 17.Asiaie R, Huang X, Farnan D, Horvath C. J. Chromatogr. A. 1998;806:251–263. doi: 10.1016/s0021-9673(98)00052-1. [DOI] [PubMed] [Google Scholar]
- 18.Dulay MT, Kulkarni RP, Zare RN. Anal. Chem. 1998;70:5103–5107. doi: 10.1021/ac9806456. [DOI] [PubMed] [Google Scholar]
- 19.Ratnayake CK, Oh CS, Henry MP. J. Chromatogr. A. 2000;887:277–285. doi: 10.1016/s0021-9673(99)01142-5. [DOI] [PubMed] [Google Scholar]
- 20.Chirica G, Remcho VT. Electrophoresis. 1999;20:50–56. doi: 10.1002/(SICI)1522-2683(19990101)20:1<50::AID-ELPS50>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 21.Tang QL, Lee ML. J. Chromatogr. A. 2000;887:265–275. doi: 10.1016/s0021-9673(99)01196-6. [DOI] [PubMed] [Google Scholar]
- 22.Fields SM. Anal. Chem. 1996;68:2709–2712. doi: 10.1021/ac951247v. [DOI] [PubMed] [Google Scholar]
- 23.Ishizuka N, Minakuchi H, Nakanishi K, Soga N, et al. High Resolut. Chromatogr. J. 1998;21:477–479. [Google Scholar]
- 24.Fujimoto C. High Resolut. Chromatogr. J. 2000;23:89–92. [Google Scholar]
- 25.Ericson C, Liao JL, Nakazato K, Hjerten S. J. Chromatogr. A. 1997;767:33–41. [Google Scholar]
- 26.Palm A, Novotny MV. Anal. Chem. 1997;69:4499–4507. [Google Scholar]
- 27.Peters EC, Petro M, Svec F, Frechet JMJ. Anal. Chem. 1997;69:3646–3649. doi: 10.1021/ac970377w. [DOI] [PubMed] [Google Scholar]
- 28.Reyes DR, Iossifidis D, Auroux PA, Manz A. Anal. Chem. 2002;74:2623–2636. doi: 10.1021/ac0202435. [DOI] [PubMed] [Google Scholar]
- 29.Auroux PA, Iossifidis D, Reyes DR, Manz A. Anal. Chem. 2002;74:2637–2652. doi: 10.1021/ac020239t. [DOI] [PubMed] [Google Scholar]
- 30.Ocvirk G, Verpoorte E, Manz A, Grassbauer M, et al. Anal.Meth. Instrument. 1995;2:74–82. [Google Scholar]
- 31.Seiler K, Fan ZHH, Fluri K, Harrison DJ. Anal. Chem. 1994;66:3485–3491. [Google Scholar]
- 32.Regnier FE, He B, Lin S, Busse J. Trends Biotechnol. 1999;17:101–106. doi: 10.1016/s0167-7799(98)01294-3. [DOI] [PubMed] [Google Scholar]
- 33.Bruin GJM. Electrophoresis. 2000;21:3931–3951. doi: 10.1002/1522-2683(200012)21:18<3931::AID-ELPS3931>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 34.Kutter JP. Trac-Trends Anal. Chem. 2000;19:352–363. [Google Scholar]
- 35.Yu C, Svec F, Frechet JMJ. Electrophoresis. 2000;21:120–127. doi: 10.1002/(SICI)1522-2683(20000101)21:1<120::AID-ELPS120>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 36.Nischang I, Svec F, Frechet JMJ. J. Chromatogr. A. 2009 doi: 10.1016/j.chroma.2009.01.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vegvari A, Guttman A. Electrophoresis. 2006;27:716–725. doi: 10.1002/elps.200500789. [DOI] [PubMed] [Google Scholar]
- 38.Schaller D, Hilder EF, Haddad PR. J. Sep. Sci. 2006;29:1705–1719. doi: 10.1002/jssc.200600169. [DOI] [PubMed] [Google Scholar]
- 39.Stulik K, Pacakova V, Suchankova J, Coufal P. J. Chromatogr. B. 2006;841:79–87. doi: 10.1016/j.jchromb.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Klodzinska E, Moravcova D, Jandera P, Buszewski B. J. Chromatogr. A. 2006;1109:51–59. doi: 10.1016/j.chroma.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 41.Qin F, Xie C, Yu Z, Kong L, et al. J. Sep. Sci. 2006;29:1332–1343. doi: 10.1002/jssc.200600030. [DOI] [PubMed] [Google Scholar]
- 42.Wistuba D, Schurig V. J. Sep. Sci. 2006;29:1344–1352. doi: 10.1002/jssc.200600114. [DOI] [PubMed] [Google Scholar]
- 43.Preinerstorfer B, Laemmerhofer M. Electrophoresis. 2007;28:2527–2565. doi: 10.1002/elps.200700070. [DOI] [PubMed] [Google Scholar]
- 44.Eeltink S, Svec F. Electrophoresis. 2007;28:137–147. doi: 10.1002/elps.200600573. [DOI] [PubMed] [Google Scholar]
- 45.Nischang I, Tallarek U. Electrophoresis. 2007;28:611–626. doi: 10.1002/elps.200600625. [DOI] [PubMed] [Google Scholar]
- 46.Liu ZS, Zheng C, Yan C, Gao RY. Electrophoresis. 2007;28:127–136. doi: 10.1002/elps.200600544. [DOI] [PubMed] [Google Scholar]
- 47.Zhu G, Zhang L, Yuan H, Liang Z, et al. J. Sep. Sci. 2007;30:792–803. doi: 10.1002/jssc.200600496. [DOI] [PubMed] [Google Scholar]
- 48.Miksik I, Sedlakova P. J. Sep. Sci. 2007;30:1686–1703. doi: 10.1002/jssc.200700084. [DOI] [PubMed] [Google Scholar]
- 49.Scherz H, Huck CW, Bonn GK. Electrophoresis. 2007;28:1645–1657. doi: 10.1002/elps.200500917. [DOI] [PubMed] [Google Scholar]
- 50.Ou J, Dong J, Dong X, Yu Z, et al. Electrophoresis. 2007;28:148–163. doi: 10.1002/elps.200600298. [DOI] [PubMed] [Google Scholar]
- 51.Wu R, Hu L, Wang F, Ye M, et al. J. Chromatogr. A. 2008;1184:369–392. doi: 10.1016/j.chroma.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 52.Smith NW, Jiang Z. J. Chromatogr. A. 2008;1184:416–440. doi: 10.1016/j.chroma.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 53.Svec F, Tennikova TB, Deyl Z, editors. Monolithic materials: preparation, properties, and applications. Elsevier: Amsterdam; 2003. [Google Scholar]
- 54.Svec F, Huber CG. Anal. Chem. 2006;78:2100–2107. doi: 10.1021/ac069383v. [DOI] [PubMed] [Google Scholar]
- 55.Svec F. J. Sep. Sci. 2004;27:1255–1272. doi: 10.1002/jssc.200401906. [DOI] [PubMed] [Google Scholar]
- 56.Zhang YP, Ye XW, Tian MK, Qu LB, et al. J. Chromatogr. A. 2008;1188:43–49. doi: 10.1016/j.chroma.2007.10.068. [DOI] [PubMed] [Google Scholar]
- 57.Huang HY, Huang IY, Lin HY. J. Sep. Sci. 2006;29:2038–2048. doi: 10.1002/jssc.200600071. [DOI] [PubMed] [Google Scholar]
- 58.Jin W, Fu H, Huang X, Xiao H, et al. Electrophoresis. 2003;24:3172–3180. doi: 10.1002/elps.200305579. [DOI] [PubMed] [Google Scholar]
- 59.Huang HY, Lin HY, Lin SP. Electrophoresis. 2006;27:4674–4681. doi: 10.1002/elps.200600125. [DOI] [PubMed] [Google Scholar]
- 60.Huang HY, Liu YC, Cheng YJ. J. Chromatogr. A. 2008;1190:263–270. doi: 10.1016/j.chroma.2008.02.105. [DOI] [PubMed] [Google Scholar]
- 61.Huang X, Zhang S, Schultz GA, Henion JD. Anal. Chem. 2002;74:2336–2344. doi: 10.1021/ac011202w. [DOI] [PubMed] [Google Scholar]
- 62.Kucerova Z, Szumski M, Buszewski B, Jandera P. J. Sep. Sci. 2007;30:3018–3026. doi: 10.1002/jssc.200700346. [DOI] [PubMed] [Google Scholar]
- 63.Wu RN, Zou HF, Fu HJ, Jin W, et al. Electrophoresis. 2002;23:1239–1245. doi: 10.1002/1522-2683(200205)23:9<1239::AID-ELPS1239>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 64.Bedair M, El Rassi Z. J. Chromatogr. A. 2003;1013:35–45. doi: 10.1016/s0021-9673(03)01031-8. [DOI] [PubMed] [Google Scholar]
- 65.Svec F. Electrohoresis. 2008;29:1593–1603. doi: 10.1002/elps.200700569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao JL, Chen N, Ericson C, Hjerten S. Anal. Chem. 1996;68:3468–3472. doi: 10.1021/ac960261k. [DOI] [PubMed] [Google Scholar]
- 67.Ericson C, Hjerten S. Anal. Chem. 1999;71:1621–1627. [Google Scholar]
- 68.Hoegger D, Freitag R. J. Chromatogr. A. 2001;914:211–222. doi: 10.1016/s0021-9673(00)01119-5. [DOI] [PubMed] [Google Scholar]
- 69.Al-Rimawi F, Pyell U. J. Sep. Sci. 2006;29:2816–2826. doi: 10.1002/jssc.200600218. [DOI] [PubMed] [Google Scholar]
- 70.Casper P, Glockner P, Ritter H. Macromolecules. 2000;33:4361–4364. [Google Scholar]
- 71.Que AH, Palm A, Baker AG, Novotny MV. J. Chromatogr. 2000;887:379–391. doi: 10.1016/s0021-9673(00)00427-1. [DOI] [PubMed] [Google Scholar]
- 72.Que AH, Konse T, Baker AG, Novotny MV. Anal. Chem. 2000;72:2703–2710. doi: 10.1021/ac991059v. [DOI] [PubMed] [Google Scholar]
- 73.Hemstroem P, Irgum K. J. Sep. Sci. 2006;29:1784–1821. doi: 10.1002/jssc.200600199. [DOI] [PubMed] [Google Scholar]
- 74.Que AH, Novotny MV. Anal. Chem. 2002;74:5184–5191. doi: 10.1021/ac025781w. [DOI] [PubMed] [Google Scholar]
- 75.Que AH, Mechref Y, Huang Y, Taraszka JA, et al. Anal. Chem. 2003;75:1684–1690. doi: 10.1021/ac025985c. [DOI] [PubMed] [Google Scholar]
- 76.Tegeler T, Mechref Y, Boraas K, Reilly JP, et al. Anal. Chem. 2004;76:6698–6706. doi: 10.1021/ac049341b. [DOI] [PubMed] [Google Scholar]
- 77.Guryca V, Mechref Y, Palm AK, Michalek J, et al. J.f Biochem Biophys. Meth. 2007;70:3–13. doi: 10.1016/j.jbbm.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walhagen K, Gaspari M, Tjaden UR, Rozing GP, et al. Rapid Comm. Mass Spectr. 2001;15:878–883. doi: 10.1002/rcm.291. [DOI] [PubMed] [Google Scholar]
- 79.Zheng J, Shamsi SA. Anal. Chem. 2003;75:6295–6305. doi: 10.1021/ac030193j. [DOI] [PubMed] [Google Scholar]
- 80.Bandilla D, Skinner CD. J. Chromatogr. A. 2004;1044:113–129. doi: 10.1016/j.chroma.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 81.Klampfl CW. J. Chromatogr. A. 2004;1044:131–144. doi: 10.1016/j.chroma.2004.04.072. [DOI] [PubMed] [Google Scholar]
- 82.Barcelo-Barrachina E, Moyano E, Galceran MT. Electrophoresis. 2004;25:1927–1948. doi: 10.1002/elps.200305908. [DOI] [PubMed] [Google Scholar]
- 83.Okanda FM, El Rassi Z. Electrophoresis. 2006;27:1020–1030. doi: 10.1002/elps.200500766. [DOI] [PubMed] [Google Scholar]
- 84.Dong X, Dong J, Ou J, Zhu Y, et al. Electrophoresis. 2006;27:2518–2525. doi: 10.1002/elps.200500865. [DOI] [PubMed] [Google Scholar]
- 85.Tian Y, Yang F, Yang X, Fu E, et al. Electrophoresis. 2008;29:2293–2300. doi: 10.1002/elps.200700766. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Lu H, Lin X, Xie Z. Electrophoresis. 2008;29:928–935. doi: 10.1002/elps.200700600. [DOI] [PubMed] [Google Scholar]
- 87.Lin X, Wang J, Li L, Wang X, et al. J. Sep. Sci. 2007;30:3011–3017. doi: 10.1002/jssc.200700329. [DOI] [PubMed] [Google Scholar]
- 88.Zhong H, El Rassi Z. J. Sep. Sci. 2009;32:10–20. doi: 10.1002/jssc.200800546. [DOI] [PubMed] [Google Scholar]
- 89.Svec F, Hradil J, Coupek J, Kalal J. Angew. Macromol. Chem. 1975;48:135–143. [Google Scholar]
- 90.Eeltink S, Rozing GP, Schoenmakers PJ, Kok WT. J. Chromatogr. A A. 2006;1109:74–79. doi: 10.1016/j.chroma.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 91.Adalid AM, Herrero-Martinez JM, Rosello S, Maquieira A, et al. Electrophoresis. 2007;28:4120–4127. doi: 10.1002/elps.200600775. [DOI] [PubMed] [Google Scholar]
- 92.Barcelo-Barrachina E, Moyano E, Puignou L, Galcera MT. Electrophoresis. 2007;28:1704–1713. doi: 10.1002/elps.200600356. [DOI] [PubMed] [Google Scholar]
- 93.Peters EC, Petro M, Svec F, Frechet JMJ. Anal. Chem. 1997;69:3646–3649. doi: 10.1021/ac970377w. [DOI] [PubMed] [Google Scholar]
- 94.Peters EC, Petro M, Svec F, Frechet JMJ. Anal. Chem. 1998;70:2288–2295. doi: 10.1021/ac9713518. [DOI] [PubMed] [Google Scholar]
- 95.Peters EC, Petro M, Svec F, Frechet JMJ. Anal. Chem. 1998;70:2296–2302. doi: 10.1021/ac9713520. [DOI] [PubMed] [Google Scholar]
- 96.Huo Y, Schoenmakers PJ, Kok WT. J. Chromatogr. A. 2007;1175:81–88. doi: 10.1016/j.chroma.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 97.Lerma-Garcia MJ, Simo-Alfonso EF, Ramis-Ramos G, Herrero-Martinez JM. Electrophoresis. 2007;28:4128–4135. doi: 10.1002/elps.200700195. [DOI] [PubMed] [Google Scholar]
- 98.Lue H, Wang J, Wang X, Lin X, et al. J.f Pharmaceut. Biomed. Anal. 2007;43:352–357. doi: 10.1016/j.jpba.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 99.Lerma-Garcia MJ, Simo-Alfonso EF, Ramis-Ramos G, Herrero-Martinez JM. Electrophoresis. 2008;29:4603–4611. doi: 10.1002/elps.200800247. [DOI] [PubMed] [Google Scholar]
- 100.Tanret I, Mangelings D, Vander Heyden Y. Electrophoresis. 2008;29:4463–4474. doi: 10.1002/elps.200800296. [DOI] [PubMed] [Google Scholar]
- 101.Eeltink S, Hilder EF, Geiser L, Svec F, et al. J. Sep. Sci. 2007;30:407–413. doi: 10.1002/jssc.200600316. [DOI] [PubMed] [Google Scholar]
- 102.Eeltink S, Desmet G, Vivo-Truyols G, Rozing GP, et al. J. Chromatogr. A. 2006;1104:256–262. doi: 10.1016/j.chroma.2005.11.112. [DOI] [PubMed] [Google Scholar]
- 103.Bedair M, El Rassi Z. Electrophoresis. 2002;23:2938–2948. doi: 10.1002/1522-2683(200209)23:17<2938::AID-ELPS2938>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 104.Okanda FM, El Rassi Z. Electrophoresis. 2005;26:1988–1995. doi: 10.1002/elps.200500073. [DOI] [PubMed] [Google Scholar]
- 105.Bedair M, El Rassi Z. J. Chromatogr. A. 2003;1013:47–56. doi: 10.1016/s0021-9673(03)01032-x. [DOI] [PubMed] [Google Scholar]
- 106.Zhang M, El Rassi Z. J. Proteome Res. 2006;5:2001–2008. doi: 10.1021/pr060185u. [DOI] [PubMed] [Google Scholar]
- 107.Lu H, Wang J, Wang X, Wu X, et al. J. Sep. Sci. 2007;30:2993–2999. doi: 10.1002/jssc.200700220. [DOI] [PubMed] [Google Scholar]
- 108.Holdsvendova P, Coufal P, Suchankova J, Tesarova E, et al. J. Sep. Sci. 2003;26:1623–1628. [Google Scholar]
- 109.Canto-Mirapeix A, Herrero-Martinez JM, Benavente D, Mongay-Fernandez C, et al. Electrophoresis. 2008;29:910–918. doi: 10.1002/elps.200700458. [DOI] [PubMed] [Google Scholar]
- 110.Augustin V, Jardy A, Gareil P, Hennion MC. J. Chromatogr. A. 2006;1119:80–87. doi: 10.1016/j.chroma.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 111.Bandilla D, Skinner CD. J. Chromatogr. A. 2003;1004:167–179. doi: 10.1016/s0021-9673(03)00450-3. [DOI] [PubMed] [Google Scholar]
- 112.Lazar IM, Li L, Yu Y, Karger BL. Electrophoresis. 2003;24:3655–3662. doi: 10.1002/elps.200305609. [DOI] [PubMed] [Google Scholar]
- 113.Carbonnier B, Guerrouache M, Denoyel R, Millot MC. J. Sep. Sci. 2007;30:3000–3010. doi: 10.1002/jssc.200700384. [DOI] [PubMed] [Google Scholar]
- 114.Guerrouache M, Carbonnier B, Vidal-Madjar C, Millot MC. J. Chromatogr. A. 2007;1149:368–376. doi: 10.1016/j.chroma.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 115.Augustin V, Stachowiak TB, Svec F, Frechet JMJ. Electrophoresis. 2009;29:3875–3886. doi: 10.1002/elps.200700883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rohr T, Hilder EF, Donovan JJ, Svec F, et al. Macromolecules. 2003;36:1677–1684. [Google Scholar]
- 117.Rohr T, Ogeltree DF, Svec F, Frechet JMJ. Adv. Funct. Mater. 2003;13:265–270. [Google Scholar]
- 118.Hilder EF, Svec F, Frechet JMJ. Anal. Chem. 2004;76:3887–3892. doi: 10.1021/ac049732q. [DOI] [PubMed] [Google Scholar]
- 119.Hilder EF, Svec F, Frechet JMJ. J. Chromatogr. A. 2004;1044:3–22. doi: 10.1016/j.chroma.2004.04.057. [DOI] [PubMed] [Google Scholar]
- 120.Yan C, Dadoo R, Zare RN, Rakestraw DJ, et al. Anal. Chem. 1996;68:2726–2730. doi: 10.1021/ac960020c. [DOI] [PubMed] [Google Scholar]
- 121.Huber CG, Choudhari G, Horvath C. Anal. Chem. 1997;69:4429–4436. doi: 10.1021/ac970393t. [DOI] [PubMed] [Google Scholar]
- 122.Steiner F, Scherer B. J. Chromatogr A. 2000;887:55–83. doi: 10.1016/s0021-9673(00)00551-3. [DOI] [PubMed] [Google Scholar]
- 123.Rimmer CA, Piraino SM, Dorsey JG. J. Chromatogr.A. 2000;887:115–124. doi: 10.1016/s0021-9673(00)00534-3. [DOI] [PubMed] [Google Scholar]
- 124.Djordjevic NM, Fitzpatrick F, Houdiere F, Lerch G, et al. J. Chromatogr. A. 2000;887:245–252. doi: 10.1016/s0021-9673(99)01129-2. [DOI] [PubMed] [Google Scholar]
- 125.Xin BM, Lee ML. J. Microcol. Sep. 1999;11:271–275. [Google Scholar]
- 126.Pucci V, Raggi MA, Svec F, Frechet JMJ. J. Sep. Sci. 2004;27:779–788. doi: 10.1002/jssc.200401828. [DOI] [PubMed] [Google Scholar]
- 127.Maruska A, Rocco A, Kornysova O, Fanali S. J. Biochem. Biophys.Meth. 2007;70:47–55. doi: 10.1016/j.jbbm.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 128.Hilder EF, Svec F, Frechet JMJ. J. Chromatogr. A. 2004;1053:101–106. [PubMed] [Google Scholar]
- 129.Hutchinson JP, Zakaria P, Bowie AR, Macka M, et al. Anal. Chem. 2005;77:407–416. doi: 10.1021/ac048748d. [DOI] [PubMed] [Google Scholar]
- 130.Zakaria P, Hutchinson JP, Avdalovic N, Liu Y, et al. Anal. Chem. 2005;77:417–423. doi: 10.1021/ac048747l. [DOI] [PubMed] [Google Scholar]
- 131.Hutchinson JP, Hilder EF, Shellie RA, Smith JA, et al. Analyst. 2006;131:215–221. doi: 10.1039/b511398a. [DOI] [PubMed] [Google Scholar]
- 132.Hutchinson JP, Hilder EF, Macka M, Avdalovic N, et al. J. Chromatogr. A. 2006;1109:10–18. doi: 10.1016/j.chroma.2005.08.076. [DOI] [PubMed] [Google Scholar]
- 133.Nilsson C, Birnbaum S, Nilsson S. J. Chromatogr. A. 2007;1168:212–224. doi: 10.1016/j.chroma.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 134.O'Mahony T, Owens VP, Murrihy JP, Guihen E, et al. J. Chromatogr. A. 2003;1004:181–193. doi: 10.1016/s0021-9673(03)00856-2. [DOI] [PubMed] [Google Scholar]
- 135.Qu Q, Zhang X, Shen M, Liu Y, et al. Electrophoresis. 2008;29:901–909. doi: 10.1002/elps.200700409. [DOI] [PubMed] [Google Scholar]
- 136.Yang L, Guihen E, Glennon JD. J. Sep. Sci. 2005;28:757–766. doi: 10.1002/jssc.200400075. [DOI] [PubMed] [Google Scholar]
- 137.Yang L, Guihen E, Holmes JD, Loughran M, et al. Anal. Chem. 2005;77:1840–1846. doi: 10.1021/ac048544x. [DOI] [PubMed] [Google Scholar]
- 138.Malik A. Electrophoresis. 2002;23:3973–3992. doi: 10.1002/elps.200290013. [DOI] [PubMed] [Google Scholar]
- 139.Pesek JJ, Matyska MT, Cho SJ. J. Chromatogr. A. 1999;845:237–246. doi: 10.1016/s0021-9673(99)00154-5. [DOI] [PubMed] [Google Scholar]
- 140.Pesek JJ, Matyska MT, Menezes S. J. Chromatogr.A. 1999;853:151–158. doi: 10.1016/s0021-9673(99)00550-6. [DOI] [PubMed] [Google Scholar]
- 141.Pesek JJ, Matyska MT, Dawson GB, Chen JIC, et al. Anal. Chem. 2004;76:23–30. doi: 10.1021/ac0302253. [DOI] [PubMed] [Google Scholar]
- 142.Huang T, Mi JQ, Zhang XX. J. Sep. Sci. 2006;29:277–281. doi: 10.1002/jssc.200500317. [DOI] [PubMed] [Google Scholar]
- 143.Eeltink S, Svec F, Frechet JMJ. Electrophoresis. 2006;27:4249–4256. doi: 10.1002/elps.200600259. [DOI] [PubMed] [Google Scholar]
- 144.Luo Q, Yue G, Valaskovic GA, Gu Y, et al. Anal. Chem. 2007;79:6174–6181. doi: 10.1021/ac070583w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yue G, Luo Q, Zhang J, Wu SL, et al. Anal. Chem. 2007;79:938–946. doi: 10.1021/ac061411m. [DOI] [PubMed] [Google Scholar]
- 146.Luo Q, Gu Y, Wu SL, Rejtar T, et al. Electrophoresis. 2008;29:1604–1611. doi: 10.1002/elps.200700741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen JR, Dulay MT, Zare RN, Svec F, et al. Anal. Chem. 2000;72:1224–1227. doi: 10.1021/ac9911793. [DOI] [PubMed] [Google Scholar]
- 148.Yuan R, Ding G, Guo Y, Liu D, et al. Electrophoresis. 2007;28:1674–1680. doi: 10.1002/elps.200600541. [DOI] [PubMed] [Google Scholar]
- 149.Nischang I, Chen G, Tallarek U. J. Chromatogr. A. 2006;1109:32–50. doi: 10.1016/j.chroma.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 150.Chaisuwan P, Nacapricha D, Wilairat P, Jiang Z, et al. Electrophoresis. 2008;29:2301–2309. doi: 10.1002/elps.200700689. [DOI] [PubMed] [Google Scholar]
- 151.Messina A, Flieger M, Bachechi F, Sinibaldi M. J. Chromatogr. A. 2006;1120:69–74. doi: 10.1016/j.chroma.2005.11.107. [DOI] [PubMed] [Google Scholar]
- 152.Stachowiak TB, Svec F, Frechet JMJ. J. Chromatogr. A. 2004;1044:97–111. doi: 10.1016/j.chroma.2004.04.075. [DOI] [PubMed] [Google Scholar]
- 153.Faure K, Blas M, Yassine O, Delaunay N, et al. Electrophoresis. 2007;28:1668–1673. doi: 10.1002/elps.200600566. [DOI] [PubMed] [Google Scholar]
- 154.Blas M, Delaunay N, Rocca JL. J. Sep. Sci. 2007;30:3043–3049. doi: 10.1002/jssc.200700132. [DOI] [PubMed] [Google Scholar]
- 155.Augustin V, Proczek G, Dugay J, Descroix S, et al. J. Sep. Sci. 2007;30:2858–2865. doi: 10.1002/jssc.200700387. [DOI] [PubMed] [Google Scholar]