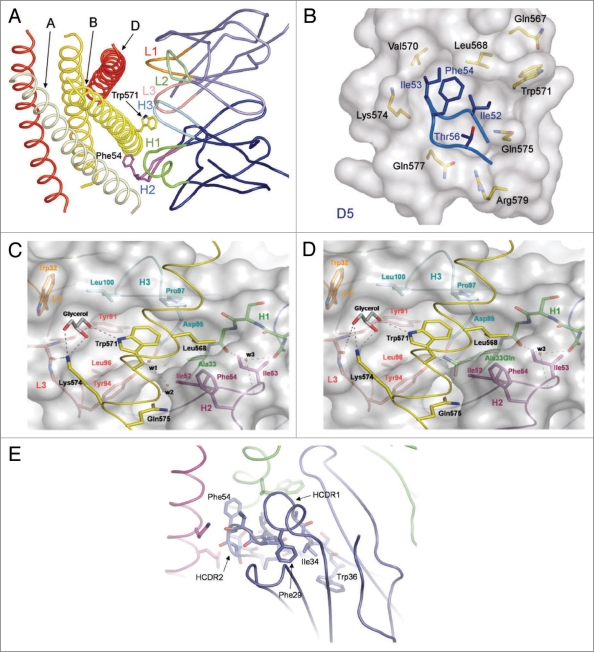
Figure 3.
Structural rationale for D5 scFv affinity maturation clones. (A) D5 CDR loops (colored and labeled) contacting the gp41 N-peptide trimeric inner core (shades of yellow and labeled). (B) D5 HC CDR2 loop entering gp41 hydrophobic pocket. Core D5 residues are labeled in blue and gp41 residues are labeled in black. (C) D5-derived ‘Trp 571’ pocket. Several residues from five D5 CDR loops surround the gp41 N-peptide. Loops and specific contact residues are colored and labeled as follows: H1 (green), H2 (purple), H3 (cyan), L1 (orange), L3 (peach). Solvent molecules are labeled in the interface and hydrogen bonds are shown as dotted lines. (D) Model of D5 mutant with alanine at position HC 33 substituted for glutamine. In this model, the water molecules w1 and w2 are replaced by the glutamine side chain such that new hydrogen bonds with gp41 Gln575, D5 HC Asp95 and D5 LC Tyr94 may exist. (E) The region of D5 HC CDR1 behind HC CDR2 contains conserved aromatic side chains (Phe29 and Trp36). In D5, the 34 position is an isoleucine, however several affinity matured clones contain a tyrosine at this residue.