Abstract
Although prostaglandin E2 (PGE2) has been shown by pharmacological and genetic studies to be important in skin cancer, the molecular mechanism(s) by which it contributes to tumor growth is not well understood. In this study we investigated the mechanisms by which PGE2 stimulates murine keratinocyte proliferation using in vitro and in vivo models. In primary mouse keratinocyte (PMK) cultures, PGE2 activated the epidermal growth factor receptor (EGFR) and its downstream signaling pathways as well as increased cyclic AMP (cAMP) production and activated the cAMP response element binding protein (CREB). EGFR activation was not significantly inhibited by pretreatment with a c-src inhibitor (PP2), nor by a protein kinase A inhibitor (H-89). However, PGE2-stimulated extracellularly-regulated kinase1/2 (ERK1/2) activation was completely blocked by EGFR, ERK1/2 and phosphatidylinositol 3-kinase (PI3-K) pathway inhibitors. In addition, these inhibitors attenuated the PGE2-induced proliferation, nuclear factor-κB (NF-κB), activator protein-1 (AP-1) and CREB binding to the promoter regions of the cyclin D1 and vascular endothelial growth factor (VEGF) genes and expression of cyclin D1 and VEGF in PMKs. Similarly, in vivo, we found that wild type (WT) mice treated with PGE2 and untreated COX-2 overexpressing transgenic mice had higher levels of cell proliferation and expression of cyclin D1 and VEGF, as well as higher levels of activated EGFR, NF-κB, AP-1 and CREB, than vehicle-treated WT mice. Our findings provide evidence for a link between COX-2 overexpression and EGFR-, ERK-, PI3-K-, cAMP-mediated cell proliferation, and the tumor promoting activity of PGE2 in mouse skin.
Introduction
Prostaglandins (PGs) have been associated with many normal and pathophysiological responses, including ovulation, vessel contraction/relaxation, renal filtration, gastrointestinal protection, angiogenesis, increased proliferation, tumorigenesis and immune suppression (1–4). Prostaglandin production is regulated by the enzyme cyclooxygenase (COX), which catalyzes the conversion of arachidonic acid to PGH2 through a two-step process involving distinct cyclooxygenase and peroxidase activities (5). Two isoforms of COX have been identified, i.e., COX-1 and COX-2. COX-1 is constitutively expressed in most tissues and cell types and mediates tissue homeostasis, whereas COX-2 is induced by a variety of mitogenic and inflammatory factors and is highly expressed in most epithelial cancers including those of skin (6).
In the context of epidermal keratinocytes, there are a number of observations suggesting that prostaglandin E2 (PGE2) is a versatile eicosanoid that plays a key role in normal skin homeostasis, although it can also act as a tumor promoter, controlling many of the behaviors typical of cancer cells (7–9). Moreover, several studies showed that PGE2 production is induced by numerous stimuli including known skin tumor promoters such as UV irradiation (10, 11) and 12-O-tetradecanoylphorbol-13-acetate (TPA) (12, 13). In addition, it has been suggested that at least some of the tumor-promoting effects of these agents are mediated by stimulation of PGE2 production (1, 7, 9, 13, 14). The requirement of high PGE2 levels for skin tumor development is further suggested by studies in which inhibitors of COX had strong chemopreventive activity (15). In addition, strong support for a role of PGs in skin tumor promotion comes from initiation-promotion studies using COX-1 and COX-2 deficient mice, in which a 70–80% reduction in papilloma number, compared with WT mice was observed in each isoform deficient mouse (16). In support of above observations, Rundhaug et al. (17) found that endogenous PGE2 in K14.COX-2 transgenic mice has tumor promoting activity. However, the role of PGE2 in keratinocyte function is still not entirely clear. While prostaglandins have not been shown to be involved in normal murine keratinocyte proliferation in vivo, PGE2 was found to be a required co-mitogen for phorbol ester-elicited hyperproliferation and after mechanical wounding (13, 18). In vitro, however, several studies suggested an involvement of PGs even in unstimulated proliferation. In human keratinocytes, a correlation between endogenous PGE2 and DNA synthesis was observed. Inhibition of proliferation by indomethacin can be overcome by exogenous addition of PGE2, strongly suggesting that PGE2 is a growth-promoting factor for the epidermis (19, 20). Collectively, these studies suggest that keratinocyte proliferation is highly regulated by PGs; an understanding of its regulation could potentially offer targets for the prevention of pathologies.
PGE2 exerts its actions either in an autocrine or paracrine fashion via binding to four Eprostanoid (EP) receptor subtypes, termed EP1, EP2, EP3 and EP4, that differ in ligand-binding affinity, tissue distribution, and coupling to intracellular signal transduction pathways (21). All four PGE2 receptors were found to be present in normal human and mouse epidermis (22, 23); however, to date there is limited information on the role of the EP receptors, and/or receptor-mediated signal transduction pathways involved in keratinocyte proliferation. Recently, we reported that the EP2 receptor, but not the EP3 receptor, plays a significant role in the protumorigenic action of TPA in skin tumor development (23). Additionally, it has been shown that COX-2 and the PGE2 receptors EP1, EP2, and/or EP4 are important for UV-induced skin carcinogenesis (reviewed in 24). Another study showed that the EP2 receptor promotes squamous cell carcinoma development through the epidermal growth factor receptor (EGFR)/inducible nitric oxide synthase/extracellular signal–regulated kinase 1/2 (ERK1/2) pathways (25). However, to date there are no reports showing a direct effect of PGE2 on keratinocyte proliferation.
Skin cancer is the most common form of cancer and the incidence is rising steadily. The average increase in new skin cancer cases has been around 3% to 8% per year since the 1960s, with approximately one million new cases diagnosed each year in the United States (26). Thus, improving our understanding of effective skin cancer chemoprevention can have a large impact on health. Work to date has focused on inhibition of cyclooxygenases as an approach to skin cancer chemoprevention since selective COX-2 inhibition can suppress tumor formation in chronically UV-irradiated mice (15). However, recent studies in humans have shown that treatment with selective COX-2 inhibitors increases the risks of cardiovascular events (27). Thus, a better understanding of the consequences of PGE2-mediated signaling in skin is needed to refine our ability to predict the risks and benefits of chemoprevention targeted to PGE2-mediated cellular functions.
The current study was designed to look at the effect of PGE2 on keratinocyte proliferation using in vitro and in vivo mouse models and to delineate the definitive mechanism(s) through which PGE2 mediates these effects. Here we report that in primary mouse keratinocytes (PMKs), PGE2 rapidly induces phosphorylation of EGFR, as well as activation of c-src, Ras, mitogen-activated protein kinases (MAPKs) and phosphatidylinositol-3-kinase (PI3-K)/Akt pathways. As expected, we found that PGE2 can also activate the cyclic AMP (cAMP)/protein kinase A (PKA) pathway. Both the EGFR/MAPK and cAMP/PKA pathways are needed for PGE2-elicited cell proliferation. We further show that PGE2 increased binding of cAMP response element binding protein (CREB), activator protein-1 (AP-1) and nuclear factor-κB (NF-κB) to the promoter regions of the cell growth regulatory genes, cyclin D1 and vascular endothelial growth factor (VEGF), and induced the expression of these genes. These findings suggest that PGE2 may promote the growth of tumors by activating several signaling pathways that lead to the expression of genes involved in proliferation and other tumor promotion processes.
Results
PGE2 induces keratinocyte proliferation in vitro
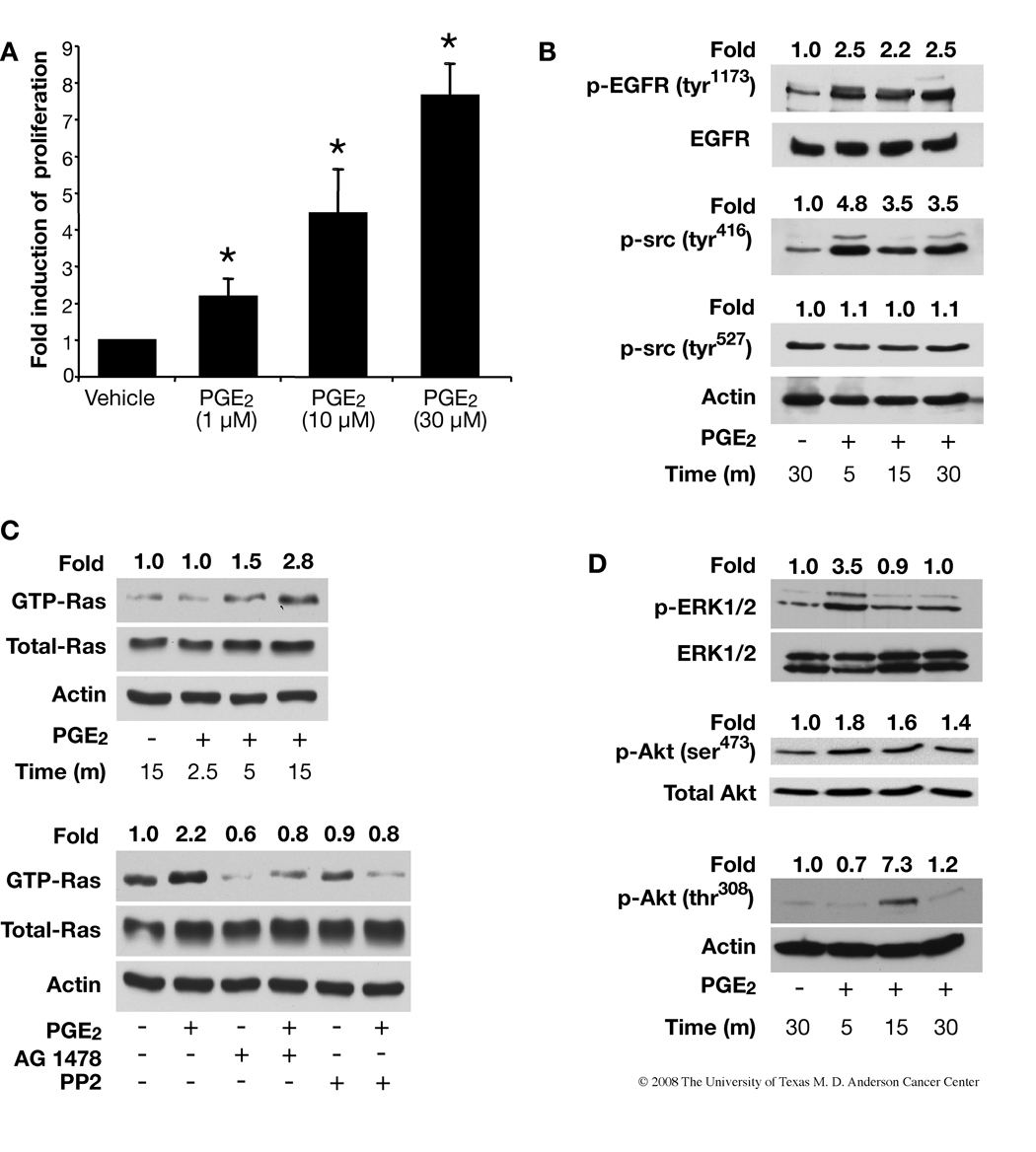
Because PGE2-induced keratinocyte proliferation may be one of the key mechanisms by which PGE2 promotes skin tumorigenesis, we investigated whether PGE2 would affect the proliferation of PMKs. As shown in Fig. 1A, DNA synthesis was stimulated in a dose-response manner following 20 h of PGE2 treatment. One, 10 and 30 µM PGE2 increased DNA synthesis by 2.2, 4.5 and 7.6 fold respectively (Fig. 1A).
FIGURE 1.
Effect of PGE2 on keratinocyte proliferation and EGFR, Ras- ERK1/2, and Akt signaling pathways in primary mouse keratinocytes (PMKs). A. PGE2 increases keratinocyte proliferation in a dose-dependent manner. PMKs from WT mice were treated with PGE2 (10–30 µM) for 20 h and pulsed with (3H)-thymidine for 2 h before harvest. The (3H)-thymidine incorporated by PMKs was measured and normalized to protein concentration. Data are presented as fold induction of specific activity. At least 2 independent experiments were done, with triplicates for each treatment group; data from a representative experiment are shown as means±SD. *p<0.05, significant when compared to the vehicle-treated group. B. Phosphorylation of EGFR and c-src was determined by western blotting using anti-p-EGFR (tyr1173) and anti-p-src (tyr416 and tyr527) antibodies. Blots were reprobed with anti-EGFR or anti-actin antibody for loading control. C. Top panels, PMKs were treated with PGE2 (10 µM) for the indicated times after serum-starvation for 24 h. GTP-bound Ras was affinity-precipitated and detected by western blotting using a pan-Ras antibody. Total Ras protein and actin show equal protein in each sample. Bottom panels, Effect of an EGFR inhibitor (AG1478) or c-src inhibitor (PP2) on PGE2-induced GTP-Ras activation. The PMKs were pretreated with the indicated inhibitors for 30 min after serum starvation for 24 h and then incubated with PGE2 (10 µM) for 5 min. Ras activation was measured as described in experimental procedures. D. PGE2 (10 µM) induces phosphorylation of ERK1/2 and Akt (ser473 and thr308) in PMKs after PGE2 treatment for the indicated time points. The same blots were reprobed with anti-ERK1/2 or anti-actin antibody for loading controls. Quantitation of the intensities of the bands were determined by densitometry and the relative ratios of the activated Ras, EGFR, c-src, ERK1/2 and Akt to total Ras, EGFR, ERK1/2 or actin were normalized to the vehicle-treated samples and are shown above each lane.
PGE2 activates EGFR-Ras-MAPK and Akt signaling pathways
Recently there has been a concerted effort to understand the signaling cascades from G-protein coupled receptors (GPCRs) to events typically thought to be associated with growth factor stimulation, such as the PI3-K/Akt and Ras/Raf/MEK pathways (28–30). Here, we investigated the effect of PGE2 on activation of EGFR, Ras, Akt, c-src and MAPKs. When PMKs were treated with 10 µM PGE2, we found that EGFR (tyr1173) and c-src (tyr416) were activated 2.5 and 4.8 over vehicle-treated cultures after 5 min, respectively (Fig. 1B). To show that PGE2 can induce endogenous Ras (wild type) activity, PMKs were treated with PGE2 for various times following serum starvation and subjected to a Ras activity assay. We found PGE2 increased Ras activation 2.8 fold over control; all the samples exhibited similar levels of total Ras protein (Fig. 1C). In addition, we found that an EGFR specific inhibitor, AG1478, and c-src inhibitor, PP2, blocked PGE2-induced Ras activity, which suggests that EGFR and c-src are upstream of Ras (Fig. 1C).
Because the MAPK cascade is downstream from EGFR and Ras, we next examined whether PGE2 could activate MAPK signaling (Fig. 1D). PMKs were treated with PGE2, followed by western blotting with antibodies that recognize the phosphorylated (activated) forms of ERK1/2, p38 and Jun-amino-terminal kinase/stress-activated protein kinase (JNK/SAPK). As shown in Fig. 1D, PGE2 enhanced phosphorylation of ERK1/2 (3.5 fold) 5 min after treatment, but there was no change in the phosphorylation of p38 or JNK/SAPK, other MAPK family members in primary keratinocytes (data not shown). We further assessed the effect of PGE2 on another signaling molecule downstream of EGFR, Akt. As shown in Fig. 1D, Akt (ser473) becomes activated (1.5 fold) after 5 min and remains activated (~2 fold) for 30 min, but Akt (thr308) becomes activated (7.3 fold) at 15 min and returns to close to control levels (1.2 fold) by 30 min. We measured phosphorylation of both sites (ser473 and thr308) of Akt because phosphorylation of thr308 in the activation loop of the kinase domain and ser473 in the C-terminal regulatory region are both required for maximal activation of Akt (31). These results show that PGE2 also stimulated Akt activation in a time-dependent manner.
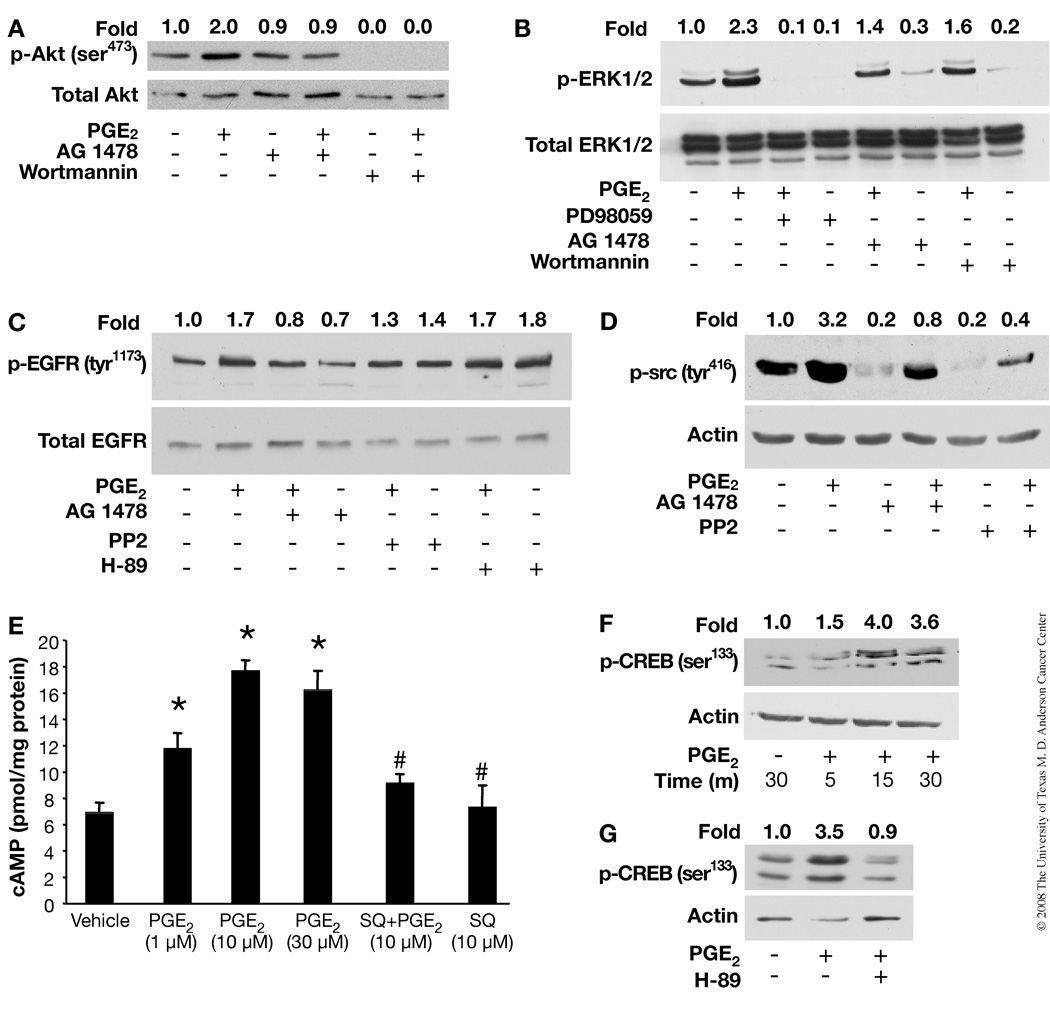
To determine the relative sequence of PGE2 signaling, the effect of pathway-specific inhibitors on the activation of various potential intermediates were examined. Preincubation of PMKs with the EGFR kinase inhibitor, AG1478, caused a complete inhibition of PGE2-induced activation of Akt, while the PI3-K inhibitor, wortmannin, completely abrogated both basal and PGE2-induced activation of Akt (Fig. 2A). Both of these results were not unexpected as EGFR is known to activate PI3-K, and PI3-K is upstream of Akt. Similarly, the ERK inhibitor, PD98059, completely blocked basal and PGE2-induced activation of ERK1/2 (Fig. 2B). On the other hand, wortmannin as well as AG1478, only partially blocked PGE2-induced ERK1/2 activation, which may be the result of their strong inhibition of basal ERK1/2 levels (Fig. 2B). Taken together, these results suggest that the PGE2-induced activation of Akt is mediated via activation of EGFR, while ERK1/2 may be activated in part through an EGFR-independent mechanism.
FIGURE 2.
Effect of pharmacological inhibitors on PGE2-induced cAMP production and EGFR, c-src, ERK1/2, Akt and PKA/ CREB signaling pathways in PMKs. PMKs were serum starved for 24 h prior to treating with vehicle or PGE2 (10 µM) for 5 min. Pathway-specific inhibitors were added 30 min before PGE2 treatment. Western blots of proteins from whole cell lysates were performed with antibodies to the proteins indicated. A. Akt and EGFR inhibitors inhibit PGE2-induced Akt activation. Both AG1478 (EGFR inhibitor) and wortmannin (Akt inhibitor) blocked PGE2-stimulated phosphorylation of Akt (ser473). B. Effect of MEK, EGFR, and Akt inhibitors on ERK1/2 activation. PD98059 (MEK/ERK inhibitor) completely blocked PGE2-stimulated ERK1/2 phosphorylation, while AG1478 and wortmannin were only partially effective. C. Effect of EGFR, c-src, and PKA inhibitors on EGFR activation. AG1478 as expected blocked PGE2-stimulated EGFR phosphorylation (tyr1173), while PP2 (c-src inhibitor) and H-89 (PKA inhibitor) had little or no effect. D. EGFR and c-src inhibitors block PGE2-induced c-src activation. Both AG1478 and PP2 completely inhibit PGE2-stimulated phosphorylation of c-src (tyr416). E. PGE2 induces cAMP production. Serum-starved PMKs were treated with PGE2 (0–30 µM) for 30 min with or without a 20 min pretreatment with SQ 22,536 (10 µM), an adenylate cyclase inhibitor. The mean (±SD) levels of cAMP from triplicate samples are expressed as pmol/mg protein. *p<0.05, significant when compared to the vehicle-treated group and #p<0.05, significant when compared to the PGE2 (10 µM)-treated group. F. PGE2 induces CREB activation. Serum-starved PMKs were treated with vehicle or PGE2 (10 µM) for the indicated times. PGE2 stimulated phosphorylation of CREB (ser133) maximally at 15 min. G. PKA inhibitor blocks PGE2-induced CREB activation. Pretreatment of PMKs with H-89 for 30 min prior to PGE2 (10 µM) treatment for 10 min inhibited phosphorylation of CREB (ser133). Quantitation of the intensities of the bands was determined by densitometry and the relative ratios of the activated Ras, EGFR, c-src, ERK1/2 or Akt to total Ras, EGFR, ERK1/2 or actin was normalized to the vehicle samples and are shown above each lane.
Effect of c-src and PKA inhibitors on activation of EGFR
Although several reports have demonstrated that activation of the c-src family of non-receptor tyrosine kinases is involved in GPCR-mediated signaling, the position of c-src in signaling pathways remains unknown (32). We examined the effect of PP2 (c-src inhibitor) and AG1478 on PGE2-induced phosphorylation of the EGFR. As shown in Fig. 2C, pretreatment of PMKs with PP2 does not significantly affect PGE2-induced phosphorylation of EGFR, while pretreatment with AG1478 blocked the PGE2-induced phosphorylation of the EGFR as expected. Interestingly, pretreatment of PMKs with either EGFR or c-src inhibitor strongly inhibited the basal level of c-src phosphorylation, suggesting that the inhibition of PGE2-induced c-src activation by these inhibitors is probably the result of reduced basal levels (Fig. 2D). Moreover, the observation that PGE2 significantly activates c-src (Fig. 1B), while c-src inhibition has little effect on EGFR activation (Fig. 2C), suggests that c-src is downstream of EGFR. There was no change in EGFR activation with the PKA inhibitor, H-89 (Fig. 2C).
PGE2-stimulated cAMP formation and phosphorylation of CREB in vitro
PGE2 activates different signaling pathways depending on the receptor to which it binds. Our recent findings (33) show that EP2 is a major PGE2 receptor, which upon ligand binding, activates adenylate cyclase with subsequent synthesis of cAMP and activation of PKA. To determine whether PGE2 increased cAMP production and thereby activated the PKA pathway and CREB-mediated transcription in our experiments, we measured cAMP levels in cultured PMKs following PGE2 treatment. The concentrations of cAMP were significantly higher (2.5 fold) in cells after PGE2 treatment than in cells without exogenous PGE2 (Fig. 2E). As expected, PGE2-induced cAMP production was inhibited in keratinocytes treated with SQ 22,536, an adenylate cyclase inhibitor. Next, we examined the effect of PGE2 on phosphorylation of ser133 of CREB. As shown in Fig. 2F, we found that PGE2 treatment can cause the phosphorylation of ser133, approximately 4 fold after 15 and 30 min. As depicted in Fig. 2G, phosphorylation of CREB can be completely blocked by H-89. These results indicate that in PMKs, PGE2 also activates the cAMP/PKA signaling pathway.
Effect of signaling inhibitors on PGE2-induced binding of transcription factors CREB, NF-κB and AP-1 to DNA
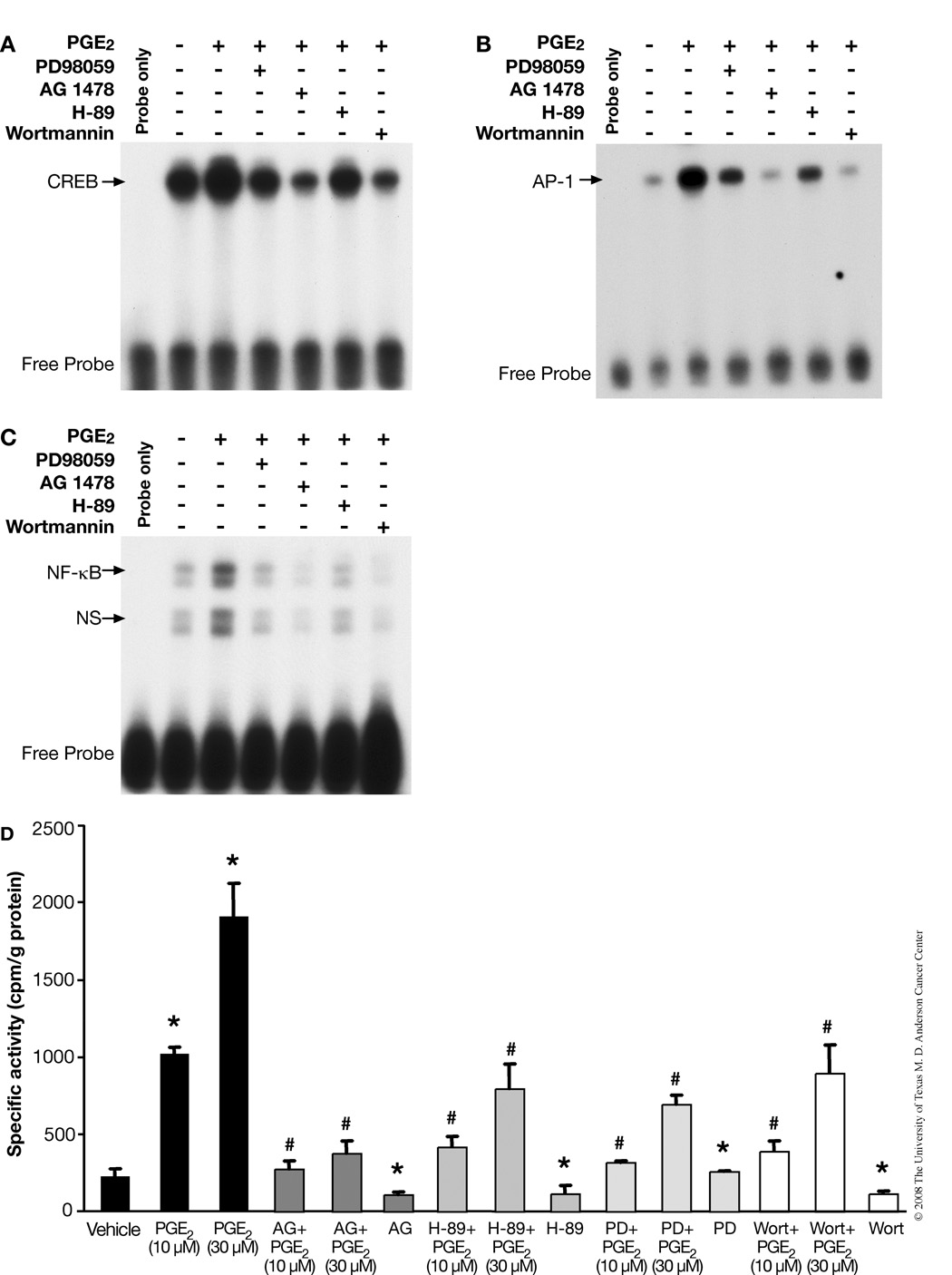
Because we observed that PGE2 can induce multiple mitogenic signaling pathways, we next assessed the effect of PGE2 and some pathway-specific inhibitors on the DNA binding activity of CREB, NF-κB and AP-1. As shown in Fig. 3A, PGE2 enhanced CREB DNA-binding activity, while AG1478, PD98059 and wortmannin inhibited PGE2-induced DNA binding activity significantly. Surprisingly, we found that there was much lower inhibition with H-89 (PKA inhibitor) compared to AG1478 and wortmannin. Similar results were seen with AP-1 (Fig. 3B) and with NF-κB (Fig. 3C) binding to DNA.
FIGURE 3.
PGE2-induces activation of CREB, AP-1 and NF-κB transcription factors in PMKs. PMKs were incubated with vehicle (control, lane 2) or with PGE2 (10 µM) (lanes 3–7) for 15 min. To demonstrate the effect of phamacological inhibitors on PGE2-induced transcription factor-binding, PMKs were treated with PD98059, AG1478, H-89 or wortmannin for 30 min prior to PGE2 treatment. Nuclear extracts were subjected to electrophoretic mobility shift assay analysis, as described in experimental procedures. A. PGE2-induced CREB activation in PMKs. The arrow indicates the specific binding of CREB to its consensus oligonucleotide. B. PGE2-induced AP-1 activation in PMKs. The arrow indicates the specific binding of AP-1 to its consensus oligonucleotide. C. PGE2-induced NF-κB activation in PMKs. The arrows indicate the specific binding of NF-κB to its consensus oligonucleotide (upper arrow) and non-specific (ns) binding (lower arrow). D. EGFR, ERK1/2, PKA/CREB and PI3-K/Akt signaling cascades are involved in PGE2-stimulated cell proliferation. PMKs were treated with various kinase inhibitors 30 min prior to PGE2 (10 µM) treatment for 20 h and pulsed with (3H)-thymidine 2 h before harvest. The (3H)-thymidine incorporated by PMKs was measured in triplicate samples and normalized to protein concentration as described in experimental procedures. Representative data from at least 2 independent experiments are presented as the means ± SD. *p<0.05, significant when compared to vehicle-treated groups and #p<0.05, significant when compared to PGE2-treated groups.
Role of EGFR, MAPK, PI3-K and cAMP/PKA signaling pathways on PGE2-induced cell proliferation
Our above findings suggest that PGE2 can activate EGFR, MAPK, PI3-K and cAMP/PKA signaling pathways and that it can induce cell proliferation in a dose dependent manner. Next, to determine whether these signaling pathways are responsible for PGE2-induced cell proliferation, cultures of PMKs were incubated with various signaling inhibitors for 30 min before PGE2 treatment and DNA synthesis measured. As shown in Fig. 3D, the EGFR inhibitor AG1478 completely blocked PGE2-induced cell proliferation. On the other hand, ERK1/2, PKA, and PI3-K inhibitors also blocked PGE2-induced cell proliferation, but not to the same extent as the EGFR inhibitor. Here, it is important to note that the PKA inhibitor H-89 does not inhibit the phosphorylation of EGFR (Fig. 2C), but can partially block PGE2-induced cell proliferation.
PGE2-induced cyclin D1 and VEGF promoter activity and mRNA expression in PMKs
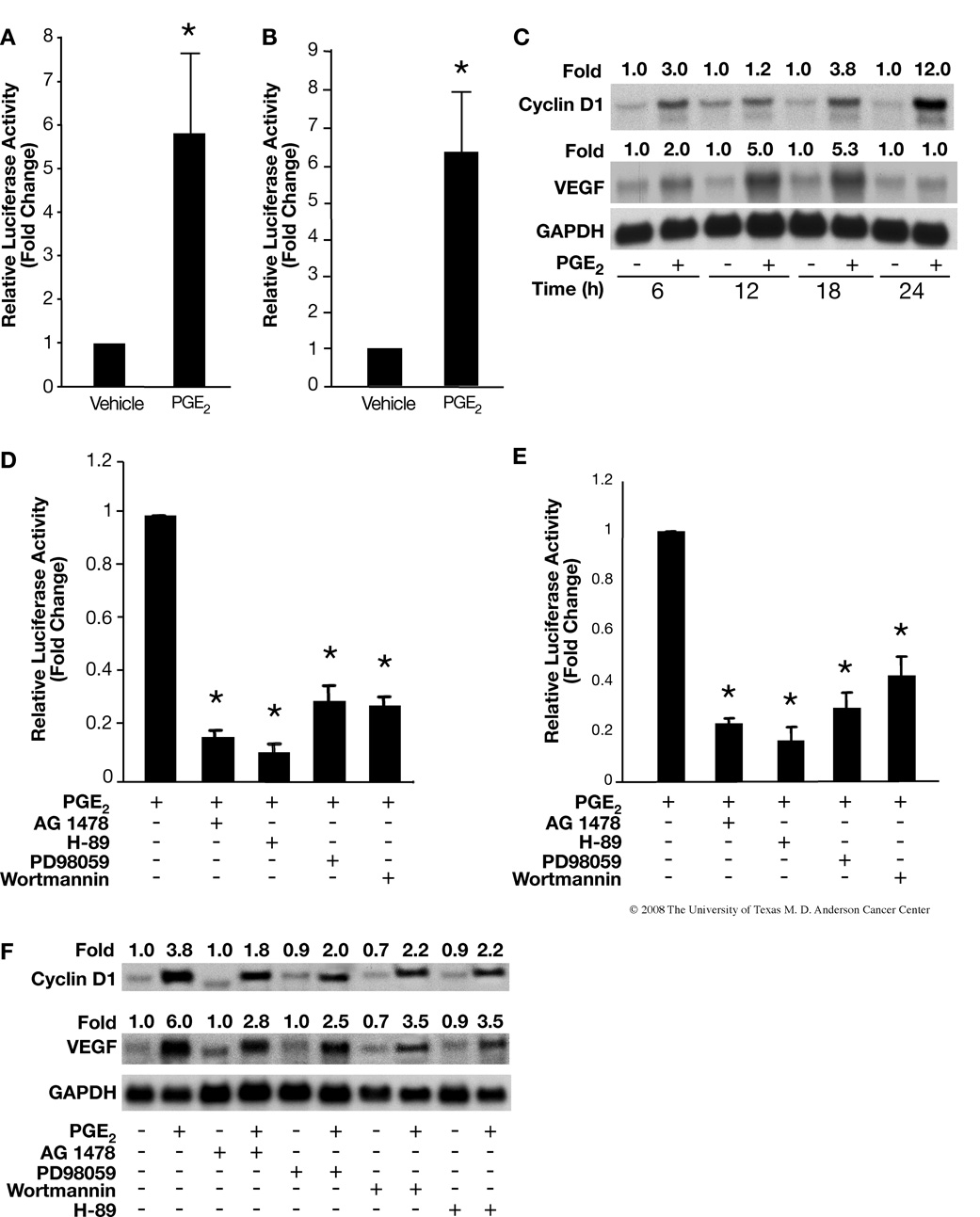
To determine whether cyclin D1 and/or VEGF genes are involved in PGE2-induced keratinocyte proliferation, as shown in Figs. 4A and B, PMKs were transiently transfected with luciferase reporter constructs containing cyclin D1 and VEGF promoters. Treatment with PGE2 for 24 h enhanced cyclin D1 as well as VEGF promoter activities. Next, we looked at the effect of PGE2 on mRNA levels of cyclin D1 and VEGF in vitro, and found maximum expression of cyclin D1 (12 fold induction) and VEGF (5 fold induction) after 24 h and 12–18 h of PGE2 treatment, respectively (Fig. 4C).
FIGURE 4.
PGE2 up-regulates cyclin D1 and VEGF expression via activation of EGFR, MAPK, cAMP/CREB and/or PI3-K/Akt cascade. PMKs were transiently transfected with cyclin D1 (A) or VEGF (B), promoter luciferase reporter constructs and CMV-β-gal plasmid followed by treatment with vehicle or PGE2 (10 µM) for 24 h. Data are presented as fold induction of relative luciferase activity. Representative data from at least 2 independent experiments using triplicates for each treatment group are presented as the means ± SD. *p<0.05, significant when compared to the vehicle treatment group. C. Effects of PGE2 on mRNA expression of cyclin D1 and VEGF. PMKs were treated with PGE2 (10 µM) for the indicated time points. RNA was isolated from the PMKs and northern blots were hybridized sequentially with cDNA probes for cyclin D1, VEGF and GAPDH (loading control). Quantitation of the intensities of the bands was determined by densitometry and the ratios of cyclin D1 and VEGF to GAPDH are shown above each lane. D and E, Specific inhibitors block PGE2 induction of cyclin D1 (D) and VEGF (E), promoter activities. Cells were pretreated with inhibitors for 30 min before the PGE2 (10 µM) treatment. Data are presented as fold induction of relative luciferase activity. Representative data from at least 2 independent experiments using triplicates for each treatment group are presented as means ± SD. *p<0.05, significant when compared to PGE2 treated group. F. Effect of specific inhibitors on PGE2-induced mRNA expression of cyclin D1 and VEGF. PMKs were pretreated with inhibitors for 30 min before the PGE2 (10 µM) treatment for 18 h. RNA was isolated and probed as (C). Quantitation of the intensities of the bands were determined by densitometry and the ratios of cyclin D1 and VEGF to GAPDH are shown above each lane.
Role of EGFR, MAPK, PI3-K and cAMP/PKA signaling pathways on PGE2 induction of cyclin D1 and VEGF
To further elucidate the molecular mechanism by which PGE2 enhanced cyclin D1 and VEGF transcription and mRNA expression, we investigated the effect of EGFR, ERK1/2, PI3-K and PKA inhibitors on PGE2-induced cyclin D1 and VEGF promoter activities and mRNA expression. We incubated PMKs in the presence of EGFR, ERK1/2, PI3-K and PKA inhibitors for 30 min before PGE2 treatment. As shown in Figs. 4D and E, the pharmacological inhibitors significantly reduced PGE2-induced cyclin D1 and VEGF promoter activities. Next, we were interested in the effect of these pathway inhibitors on PGE2-induced expression of cyclin D1 and VEGF mRNA. As shown in Fig. 4F (upper panel), cyclin D1 mRNA levels were significantly decreased with inhibition of either EGFR, PKA, ERK or PI3-K. The levels of VEGF mRNA (Fig. 4F, lower panel) were also decreased significantly, although not as completely as cyclin D1 mRNA levels.
Binding of CREB, AP-1 and NF-κB to the promoter region of cyclin D1 and VEGF
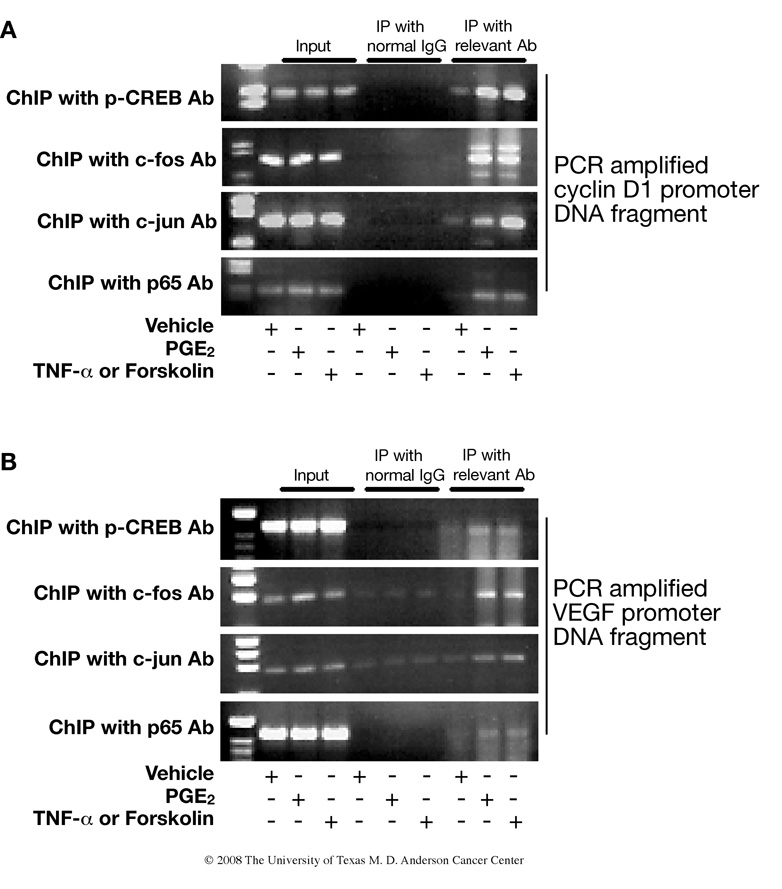
To provide further evidence that CREB, NF-κB, or AP-1 regulate cyclin D1 and VEGF promoter activity after PGE2 treatment, and to show that the interaction of CREB, NF-κB, and AP-1 with endogenous cyclin D1 and VEGF promoters occurs in intact cells, we performed chromatin immunoprecipitation (ChIP) assays. The ChIP data revealed that CREB, NF-κB, and AP-1 physically bound to the promoter region of cyclin D1 and that binding was significantly enhanced by PGE2 treatment (Fig. 5A). Similar results were found for CREB, NF-κB, and AP-1 binding to the promoter region of VEGF (Fig. 5B). Tumor necrosis factor-α (TNF-α) and forskolin were used as positive controls in this experiment because TNF-α is known to be a strong inducer of NF-κB and AP-1 activity (34) and forskolin activates adenylate cyclase, which results in activation of CREB (35).
FIGURE 5.
Chromatin immunoprecipitation assay demonstrates in vivo binding of CREB, AP-1 and NF-κB to the promoter regions of cyclin D1 and VEGF. PMKs were treated with vehicle, PGE2 (10 µM), TNF-α (10 ng/ml) or forskolin (10 µM) for 30 min. Chromatin fragments were immunoprecipitated with antibodies against phospho-CREB, c-Fos, c-Jun, p65 of NF-κB or with control rabbit immunoglobulin. Chromatin-bound DNA and input (original nuclear extract) was subjected to PCR amplification using primers specific for promoter regions of cyclin D1 (A) and VEGF (B). TNF-α and forskolin were used as positive controls for induction of AP-1, NF-κB and CREB, respectively.
PGE2 can induce cell proliferation in vivo
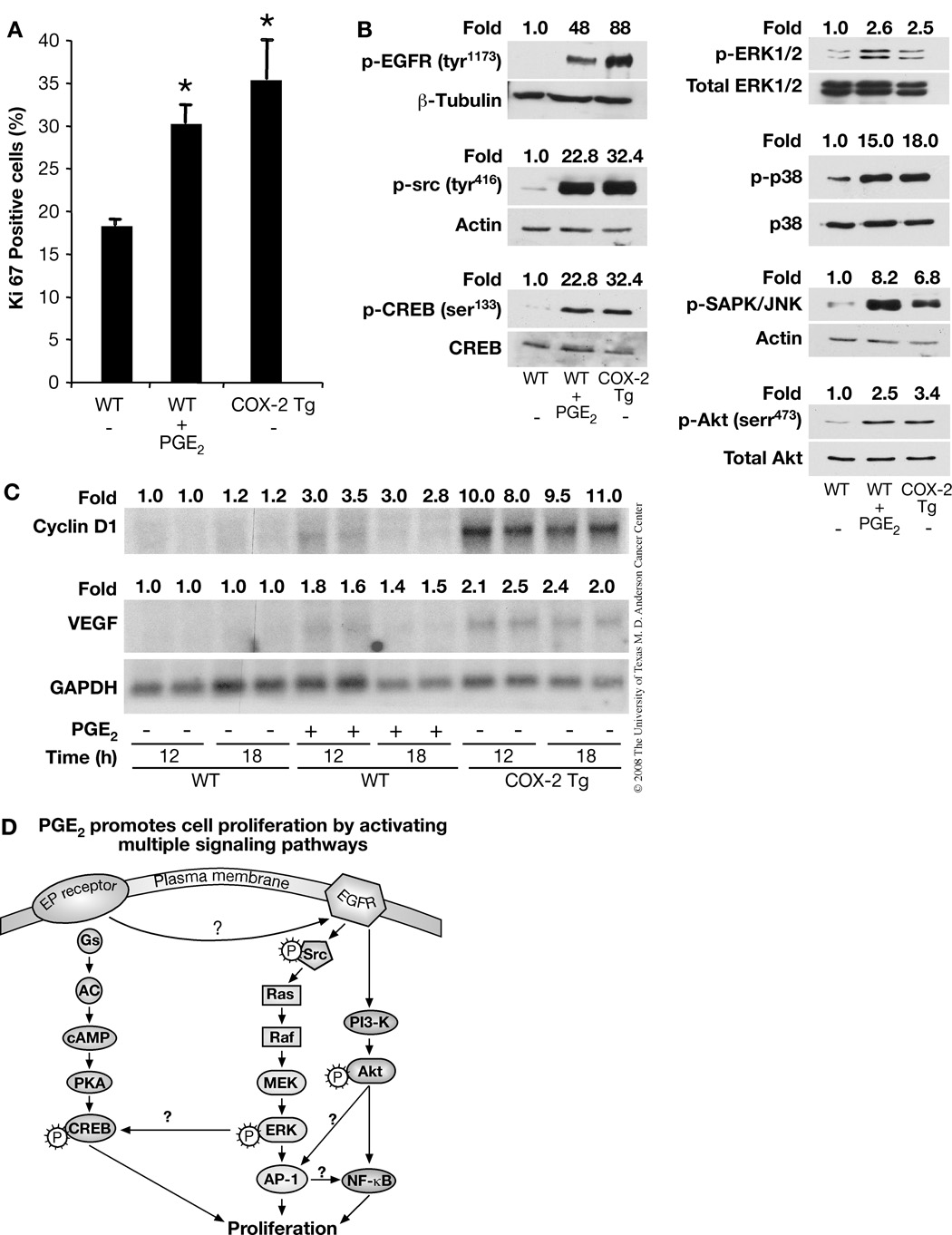
To study the role of PGE2 in the proliferation of mouse keratinocytes in vivo, we immunostained skin sections for Ki-67. Wild-type (WT) mice were topically treated with either vehicle or PGE2 (30 µg/mouse) for 18 h. To determine if endogenous PGE2 could also stimulate keratinocyte proliferation in vivo, we also immunostained skin sections from vehicle-treated K14.COX-2 transgenic mice. These transgenic mice overexpress COX-2 and have about 2-fold elevated levels of PGE2 in the epidermis (36). Our results showed that the number of Ki-67 positive epidermal cells in PGE2-treated WT mice and in vehicle-treated K14.COX-2 transgenic mice was significantly higher than in vehicle-treated WT mice (Fig. 6A), i.e., 18.3% cells were Ki-67 positive in vehicle-treated WT mice compared to 30.2% and 35.4% Ki-67 positive epidermal cells in PGE2-treated WT and vehicle-treated COX-2 transgenic mice, respectively.
FIGURE 6.
Exogenous, as well as endogenous, PGE2 triggers mitogenic signaling pathways and increases the mRNA expression of cyclin D1 and VEGF and induces cell proliferation in vivo. A. The number of Ki-67 positive basal epidermal keratinocytes were determined in vehicle- or PGE2 (30 µg/mouse) treated WT and vehicle-treated K14.COX-2 transgenic mice. The mean percentage of positive cells ± SD for each treatment group are shown. Differences between vehicle-treated WT and PGE2-treated WT or vehicle-treated COX-2 transgenic (*) mice were significant, p<0.05. B. WT mice were treated with vehicle or 30 µg PGE2, and K14.COX2 transgenic mice were treated with vehicle for 15 min before sacrifice. Epidermal lysates from 3 mice in each group were pooled together and phosphorylation of EGFR (tyr1173), c-src (tyr416), CREB (ser133), ERK1/2, p38, JNK/SAPK, Akt (ser473) and total EGFR, CREB, ERK1/2, p38, Akt or actin were analyzed by western blotting. Quantitation of the intensities of the bands shown were determined by densitometry and the relative ratios of phosphorylated forms to total EGFR, total ERK1/2, total p38, total Akt or actin were normalized to the vehicle-treated samples and are shown above each lane. This experiment was repeated without pooling lysates and gave similar results. C. Effects of exogenous and endogenous PGE2 on mRNA expression of cyclin D1 and VEGF in vivo. WT mice and K14.COX2 transgenic mice were treated as (B). These 3 groups of mice were sacrificed at the indicated times after PGE2 or vehicle treatment. RNA was isolated from the skin and northern blots were hybridized sequentially with cDNA probes for cyclin D1, VEGF and GAPDH (loading control). Quantitation of the intensities of the bands were determined by densitometry and the ratios of cyclin D1 and VEGF to GAPDH are shown above each lane. D. A schematic drawing showing the signal transduction pathways mediating PGE2-induced primary keratinocyte proliferation. See the text for details.
Activation of EGFR, c-src, CREB, MAPKs and Akt in K14.COX-2 transgenic and WT mouse skin
To determine whether the PGE2-elicited signaling events and proliferative outcome occur in vivo either after exogenous PGE2 treatment or by high endogenous levels of PGE2, WT mice (3 in each group) were treated with either vehicle or PGE2 and 3 K14.COX-2 transgenic mice were treated with vehicle only. For western blotting, epidermal lysates of each group of 3 mice were pooled together. As shown in Fig. 6B, EGFR was found to be highly phosphorylated in epidermal lysates from both PGE2-treated WT mice and vehicle-treated K14.COX-2 mice. Likewise, c-src and CREB were similarly highly phosphorylated in both the PGE2-treated WT mice and vehicle-treated COX-2 transgenic mice (Fig. 6B). The MAPKs, ERK1/2, p38 and JNK/SAPK were also highly phosphorylated in PGE2-treated WT mice, and to a somewhat lesser extent in vehicle-treated K14.COX-2 transgenic mice (Fig. 6B).
PGE2 can induce cyclin D1 and VEGF in vivo
Next, we looked at the effect of PGE2 on cyclin D1 and VEGF mRNA in vivo, and found maximum expression of cyclin D1 (3.5 fold) (Fig. 6C upper panel), and VEGF (1.8 fold, lower panel), 12 h after PGE2 treatment in WT mice. By comparison, the mRNA levels of cyclin D1 and VEGF were even higher in vehicle-treated K14.COX-2 mice than in PGE2-treated WT mice (Fig. 6C). Taken together, these results support our in vitro studies and demonstrate the similar consequence of PGE2-induced proliferation and signaling in vivo.
Discussion
PGE2 is a major product of prostanoid metabolism in keratinocytes in intact skin as well as in culture, with increased levels observed in cutaneous neoplasms (2, 9). Prostaglandin signaling is likely to be important in regulating normal epidermal growth as well as the hyperplastic response to epidermal inflammation (1, 8, 37). Moreover, in cultures of human keratinocytes, a correlation between endogenous PGE2 production and DNA synthesis was observed and inhibition of proliferation by indomethacin was countered by adding back PGE2 (19, 20). In addition, we have shown that endogenous PGE2 in K14.COX-2 transgenic mice has tumor promoting activity (17). Nonetheless, to date prostaglandins have not been shown to be involved in normal murine keratinocyte proliferation in vivo and the definitive mechanism(s) through which PGE2 mediates these effects also remains unknown. Our current study provides evidence that exogenous PGE2 can increase keratinocyte proliferation in cultures of PMKs and that exogenous, as well as endogenous PGE2 is able to induce keratinocyte proliferation in vivo. Our data suggest the mechanisms involve PGE2-induced phosphorylation of EGFR and activation of Ras-MAPKs, PI3-K/Akt and cAMP/PKA signaling pathways. Furthermore, our data revealed that PGE2 increased binding of CREB, NF-κB and AP-1 to the promoters of cyclin D1 and VEGF.
Several investigators have found that EGFR signaling is a major regulator of cell proliferation and survival in many epithelial cell types. Activation of EGFR and H-ras in the early stages of skin carcinogenesis may contribute tumor development (38). Chen et al. (2001) showed that the signaling pathway activated by EGFR is very important in modulating cell proliferation and is a necessary component for TPA-induced signal transduction associated with skin tumor promotion (39). In addition, egfr-deficient cells and potent inhibitors of EGFR, PD153035 and AG1478, blocked TPA-induced phosphorylation of ERKs, AP-1 activity, and cell transformation (39). Similarly, we also found that PGE2 caused an activation of EGFR in PMKs and in mouse skin. In addition, we also found that the EGFR inhibitor, AG1478, blocked PGE2-induced PMK proliferation. Thus, our data indicate that EGFR-signaling is involved in PGE2-induced normal PMK proliferation in culture and in mouse skin.
MAPKs constitute a superfamily of proteins that include ERK1/2, JNK1/2 and p38 kinases that play a central role in coordinating the transmission of various types of signals to the nucleus (40). Several reports suggest that aberrant activation of MAPKs play an important role in skin carcinogenesis. For example, elevated ERK1/2 activity has been detected in TPA-promoted mouse skin (41). In another study, TPA-promoted tumorigenesis was reduced in ERK-1 knockout mice (42). JNK and p38 also may play a role in carcinogenesis, acting as either tumor suppressors or as pro-oncogenic signaling molecules (43). Although JNK, p38 and ERK1/2 are often activated by different signals, these three MAPKs can modulate AP-1 activity (44). Moreover, ERK1/2 and p38 proteins have been shown to modulate NF-κB activation (41). The increased AP-1 and NF-κB activities play a very crucial role in skin carcinogenesis and have been implicated in cell proliferation, apoptosis, adhesion and inflammatory responses (45–47). In addition, the requirement of NF-κB has been demonstrated for TPA-induced transformation of JB6 epidermal cells (48). Interestingly, our study reveals that ERK1/2, p38 and JNK are also activated by PGE2 in vivo. However, in vitro, only ERK1/2, but not p38 or JNK, was activated. The reason for this in vivo/in vitro difference is not clear. Thus, our current results may be significant to PGE2-induced tumor promotion in light of a previous report that TPA- and/or UV-enhanced PGE2 synthesis, and activation of MAPKs, NF-κB and AP-1 are involved in keratinocyte proliferation (39, 46). However, previously, it was unknown whether MAPKs, AP-1 and NF-κB signaling were important for PGE2-induced keratinocyte proliferation and our data suggests that during skin tumor-promotion, PGE2 activates similar mitogenic signaling pathways and likely mediates skin tumor promotion through the same mechanisms as TPA and/or UV.
Multiple intracellular signaling pathways can be activated after prostanoid-receptor binding (25, 49, 50). The different signal transduction pathways induced by PGE2 seem to depend on the type of cells and on the subtype of GPCR involved in signaling (49). For example, in human embryonic kidney cells, PGE2 has been reported to be involved in phosphorylation of ERK1/2 through a PI3-K-dependent mechanism (49). Similarly, in squamous cell carcinoma, PGE2 initiates a cascade of events involving both c-src and PKA to activate EGFR (25). However, in our study PGE2-induced EGFR activation did not require PKA or c-src, as PKA and c-src inhibitors did not affect PGE2-induced phosphorylation of EGFR in normal PMKs. PGE2-induced ERK1/2 phosphorylation was blocked by the PI3-K inhibitor, suggesting that in normal PMKs, PGE2-induced activation of ERK1/2 was PI3-K dependent.
Furthermore, in this study, we also found that PGE2 induced c-src activation and this c-src appears to be downstream of the EGFR. Several reports suggest that c-src signaling plays a role in skin tumor promotion (51). Recently transgenic mice overexpressing either wild-type c-src or a constitutively active form developed significantly greater epidermal hyperplasia than nontransgenic mice after TPA treatment (52, 53). Thus during skin tumor promotion, it is possible that PGE2-induced c-src activation contributes to PGE2-stimulated keratinocyte proliferation.
Next, we demonstrated that PGE2 increases cyclin D1 and VEGF expression in PMKs and in mouse skin and this increased expression was significantly inhibited by EGFR, ERK1/2 and PKA inhibitors. In addition, our results showed that PGE2 induces activation of Akt and this activated Akt is involved in PGE2-induced cyclin D1 and VEGF expression, as expression was inhibited by wortmannin, an Akt pathway inhibitor. It has been suggested that hyperactivated Akt can promote cell proliferation, possibly through down-regulation of p27, as well as up-regulation of cyclin D1 (54). Furthermore, studies revealed that activated Akt and MAPKs can activate several transcription factors including CREB, AP-1 and NF-κB (46, 55–57), which bind to the regulatory elements of the promoter regions of genes such as cyclin D1 and VEGF (58, 59). The up-regulation of cyclin D1 and VEGF play an essential role in mouse skin carcinogenesis as cyclin D1 deficient mice showed resistance to tumor development (60) and transgenic mice over expressing VEGF showed accelerated tumor development (61). Moreover, studies have suggested that cyclin D1 can be induced by growth factors and TPA through activation of various signaling pathways including Ras/MAPKs, PI3-K/Akt, AP-1, CREB and NF-κB (62, 63). Similarly, reports suggest that VEGF is a potent regulator of angiogenesis and Ha-ras-mediated EGFR activation and Akt signaling are involved in the regulation of skin angiogenesis (64, 65). In skin carcinogenesis, angiogenesis occurs very early in papilloma development, establishing blood vessel networks as tumors progress (65). Furthermore, we have previously showed that overexpression of the EP2 receptor resulted in induction of vascularization and enhancement of VEGF expression after PGE2 treatment (66).
It is well known that cAMP regulates a variety of biological events, such as cell-type dependent proliferation and differentiation and that most of its effects are mediated through the cAMP–dependent PKA/CREB pathway (33, 35, 67). In this study, PGE2 induced higher levels of cAMP and increased CREB phosphorylation in PMKs and skin. Similarly the cAMP/PKA pathway was shown to be involved in the growth of colon cancer cells via induction of amphiregulin, an EGFR ligand (68). Thus, PGE2-induced proliferation of keratinocytes was associated with the increased cAMP levels and activated CREB. Moreover, recently, it has been shown that TPA increased the levels of cAMP in PMKs and in vivo, and promoted the activation of the CREB transcription factor (33). Thus, these data further support our hypothesis that some common signaling pathways are used by TPA and PGE2 to regulate keratinocyte proliferation in vitro and in vivo.
In summary, there are several noteworthy findings from our study. First, exogenous or endogenous PGE2 can induce keratinocyte proliferation both in vitro and in vivo. Second, this study provides evidence for PGE2-induced activation of CREB, NF-κB and AP-1 in PMKs and in mouse skin. Third, PGE2 can activate multiple signaling pathways including cAMP/PKA, EGFR, Ras-MAPK and PI3-K/AKT in vitro and in vivo that regulate activation of CREB, NF-κB and AP-1, as illustrated in Fig. 6D. These findings suggest that PGE2 induces keratinocyte proliferation, and likely promotes tumorigenesis as well, through a number of interrelated signaling pathways. Additional studies are needed to determine which EP receptor(s) is responsible for PGE2 activation of the various signaling pathways. Given the recently reported side effects associated with currently available COX-2 inhibitors (27), our findings suggest that an understanding of PGE2 signal transduction pathways that are critical for tumor development and growth may offer potential new targets for cancer prevention or intervention.
Materials and Methods
Animals
The K14.COX-2 transgenic mice on a FVB background were generated and maintained as previously described (36). Wild-type (WT) FVB mice were purchased from Harlan (Indianapolis, IN), and females were used in experiments at 6–8 weeks of age. All mice were maintained at Science Park and housed in an air conditioned animal facility which is Association for Assessment and Accreditation of Lab Animal Care accredited.
Cell Culture
PMKs from newborn WT mice were prepared as described by Yuspa et al. (69). Briefly, 1–2 day old newborns were euthanized and washed in 70% ethanol. The skin was stripped off and floated on 0.25% trypsin overnight at 4°C. The epidermis was separated from the dermis and chopped in Waymouth's medium (Gibco BRL, Gaithersburg, MD) containing 1.2 mM calcium and 10% fetal bovine serum. The cells were allowed to attach at 37°C in 5% CO2 for 2.5 h, and medium was then replaced with serum-free keratinocyte growth medium-2 (KGM-2) (Cambrex Bio Science, Walkersville, MD) containing 0.03 mM calcium.
Cell Proliferation
Two approaches were employed to measure keratinocyte proliferation in vitro and in vivo. To show the effect of PGE2 and pathway specific inhibitors on induction of keratinocyte proliferation in vitro, PMKs from WT mice, grown to about 80–85% confluence in six-well plates, were treated with vehicle or with 10 µM AG1478, 10 µM H-89, 10 µM PD98059, or 100 nM wortmannin (all from Calbiochem, San Diego, CA) in the presence or absence of PGE2 (10–30 µM; Cayman Chemical Co., Ann Arbor, MI). To determine the intracellular pathways involved in the control of keratinocyte proliferation, cells were cultured with 0–30 µM PGE2 for 20 h. Two hours prior to harvest, cells were pulsed with 1 µCi per ml media of [3H-methyl] thymidine ([3H]-thymidine: 79.20 Ci/mmol; PerkinElmer Life Sciences, Boston, MA). The cultures were processed as previously described (23), 3H-thymidine incorporation measured, and the values normalized to protein concentration. Protein concentration was determined with the BCA Kit (Pierce, Rockford, IL).
To assess the effect of PGE2 on keratinocyte proliferation in vivo, 6–8-wk old WT mice were shaved 2 days before treatment with acetone or PGE2 (30 µg/200 µl acetone); 6–8-week old vehicle-treated K14.COX-2 transgenic mice were also used. After 18 h of treatment, mice were euthanized and dorsal skins were removed, fixed in formalin, and processed for paraffin embedding and immunohistochemical staining with M724 antibody against Ki-67 (DAKO, Carpinteria, CA), by the Histology Core of the Science Park-Research Division. Ki-67-positive basal cells were counted in three to five randomly selected areas of each skin section and the mean percentage of Ki-67-positive cells and standard deviation (SD) of each treatment group were determined.
cAMP Analysis
cAMP measurement was carried out using an enzyme immunoassay kit from PerkinElmer Life Sciences using the nonacetylation method outlined in the manufacturer’s instructions. To determine the inhibitory effect of the adenylate cyclase inhibitor SQ 22,536 (Sigma Chemical Co., St Louis, MO) on cAMP levels, cultures were treated with 10 µM SQ 22,536 30 min prior to PGE2 treatment. Calculation of cAMP concentrations (pmol/mg protein) was based on the standard curve for each experiment.
Western Blotting
For western blotting, 2.5 × 106 PMKs were cultured for 24 h in serum–free medium in 60 mm dishes. Cells were treated with specific inhibitors for EGFR kinase (AG 1478, 10 µM) or MEK1/2 (PD 98059, 10 µM), PKA (H-89, 10 µM) or 100 nM wortmannin for 30 min prior to stimulation with 10 µM PGE2 (for the time periods specified in the figure legends) or with vehicle. All the inhibitors were purchased from Calbiochem. After stimulation with PGE2, cells were scraped and suspended in RIPA lysis buffer (150 mM NaCl, 50 mM Tris/HCl (pH 7.4), 1 mM EDTA, 0.1% SDS, 1.0% Triton X-100, 0.25% deoxycholate) plus protease and phosphatase inhibitors and centrifuged for 10 min at 14,000 rpm. The supernatant was used immediately for protein determination using a BCA Kit. For p-ERK1/2, p-p38, p-JNK/SAPK, p-Akt, p-src and p-CREB, proteins were resolved on a 10% SDS/PAGE gel, under reducing conditions. Separated proteins were electrophoretically transferred to polyvinylidene difluoride membranes and blocked with 5% non-fat dry milk or BSA in TBS containing 0.1% Tween-20 (TBST) for 1 h at room temperature. Blots were then incubated with specific primary antibodies for p-CREB, p-Akt (ser473), p-Akt (thr308), p-src (tyr416), p-p38, p-JNK/SAPK, or p-src (tyr527) (Cell Signaling Technology, Inc. Beverly, MA), p-ERK1/2, p-EGFR (tyr1173), ERK1/2, EGFR or horseradish peroxidase-conjugated actin (Santa Cruz Biotechnology, Inc. Santa Cruz, CA) overnight at 4°C. Blots were washed with TBST and subjected to corresponding horseradish peroxidase-conjugated secondary antibodies anti-mouse or anti-rabbit IgG, Amersham (Arlington Heights, IL) or anti-goat IgG, (Santa Cruz Biotechnology). Blots were washed with TBST and proteins detected with a chemiluminescence kit (Pierce, Rockford, IL). The membranes were stripped in 62.5 mM Tris HCl, pH 6.8, 100 mM 2-mercaptoethanol, 2% SDS at 68°C for 30 min, and reprobed with antibodies to total Akt, c-src, p38, JNK, or CREB (Cell Signaling), ERK1/2, EGFR, or actin (Santa Cruz Biotechnology), respectively. Blots were analyzed by densitometry using a Kodak Image Station, and normalized to total EGFR, total Akt, total ERK1/2, total p38, total CREB, or actin.
Northern Blot Analysis
Total RNA was extracted from PGE2- or vehicle-treated primary cell cultures and/or whole skins of mice with Tri-reagent (Molecular Research Center, Inc., Cincinnati, OH) following the manufacturer’s protocol. Ten µg of total RNA from each sample was separated on 1% agarose/6% formaldehyde gel, and then transferred to nylon membranes. [32P] dCTP-labeled cDNA probe for cyclin D1 or VEGF was hybridized to the blots at 65°C for 16 h. The blots were then washed to a final stringency of 0.1% SDS/0.1x NaCl/sodium citrate solution (where 1x is 0.15 M NaCl/15 mM sodium citrate) at 60°C and exposed to X-ray film at −80°C. The blots were stripped and reprobed with GAPDH cDNA as a loading control. Blots were analyzed by densitometry using a Kodak Image Station, and the values were normalized to GAPDH.
Preparation of Nuclear Extracts
Nuclear extracts were prepared as described previously (70), with the following modifications. In brief, PMKs were incubated in serum-free media for 24 h followed by the desired treatments, washed with cold PBS twice and scraped from the plates in ice-cold lysis buffer [(10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiotreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 µg/ml leupeptin, 2 µg/ml aprotinin and 10% Nonidet P-40 (NP-40)] and left on ice for 40 min. After vigorous vortexing for 10 s, homogenates were centrifuged at 14,000 rpm for 30 s. The resulting supernatant was collected as cytosolic extract. The pellet was then resuspended in nuclear extraction buffer (20 mM HEPES (pH 7.9), 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and 1 mM PMSF), vigorously rocked at 4°C for 45 min, and centrifuged for 5 min at 14,000 rpm. The supernatant (nuclear extract) thus obtained was used to assay the DNA-binding activity of CREB, AP-1 and NF-κB.
Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assays were done by standard techniques. Briefly, CREB,-AP-1,- or NF-κB-specific oligonucleotides (5 pmol) were end-labeled with [γ-32P] ATP (3,000 Ci/mmol) using T4 polynucleotide kinase (Promega, Madison, WI). Labeled double-stranded oligo probes were separated from free [γ-32P] ATP using G-25 Sephadex column. The consensus sequences of the oligonucleotides used were 5'-CTCTCTCTGACGTCAGCCAATCGA-3' and 5'-TCGATTGGCTGACGTCAGAGAGAG-3' for CREB, 5'-AGCTTCATGAGTCAGCCGGATC-3' and 5'-GATCCGGCTGACTCATGAAGCT-3' for AP-1 and 5'-AGTTGAGGGGACTTTCCCAGGC-3' and 5'-GCCTGGGAAAGTCCCCTCAACT-3' for NF-κB. Four µg protein from nuclear extracts were first incubated with 10 µl of 5x gel shift binding buffer containing 20% glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, 50 mM Tris-HCl, and 1 µg/µl poly(dI-dC):poly(dI-dC) and then with γ-32P end-labeled consensus oligonucleotide for 45 min at 37°C. The DNA-protein complexes thus formed were resolved on 6% nondenaturing polyacrylamide gels. Gels were then dried and subjected to autoradiography at −80°C.
Transient Transfections and Luciferase Assays
PMKs (1 × 106 per well) were plated in six-well plates and grown to about 60% confluence. Following replacement of medium with fresh KGM-2, cells were transfected with plasmid(s) as indicated below, using FuGENE6 transfection reagent (Roche Diagnostics, Indianapolis, IN) as described by the manufacturer. To test the effect of PGE2 on cyclin D1 and VEGF promoter activity, in one set of experiments, each well was transfected with 1 µg of a luciferase reporter construct driven by a 1745 bp cyclin D1 promoter (a kind gift from Dr. Chris Albanese, The Albert Einstein Cancer Center, New York, NY). In another set of experiments, PMKs were transfected with 1 µg of a luciferase reporter construct driven by a 1336 bp VEGF promoter (a kind gift from Dr. Lee M. Ellis, M. D. Anderson Cancer Center, Houston, Texas). All cultures were co-transfected with CMV-β-galactosidase (β-gal) plasmid (Clontech Laboratories, Inc., Mountain View, CA) to control for transfection efficiency. After 16 h, transfected cells were treated for 24 h with either vehicle or PGE2. Luciferase activity was measured using the Luciferase Assay System (Promega), and β-gal activity was measured using the Galacto-Light™ assay kit (Tropix, Bedford, MA). Light from either assay was detected by a luminometer (Tropix). The protein concentration of each cell lysate was quantified by the BCA protein assay. Luciferase activity was normalized to β-gal activity and protein concentration and then expressed as relative luciferase activity.
Ras Activation Assays
Ras activity was measured using a Ras Activation Assay Kit (Upstate Biotechnology, Inc., Lake Placid, NY) following the manufacturer's instructions. In brief, PMKs were stimulated with PGE2 at the indicated concentrations and for the indicated times. Cells were washed twice with ice-cold PBS and lysed in Mg2+ lysis/washing buffer (from kit) containing protease inhibitor cocktail tablets (Roche) for 15 min at 4°C. Cell lysates were centrifuged at 1,000 × g for 20 min. The protein concentrations of the supernatants were then determined with the BCA Kit. Equal amounts of sample (500 µg) were immediately affinity-precipitated using 20 µg of recombinant glutathione S-transferase-c-Raf-1 Ras binding domain fusion protein-conjugated glutathione-Sepharose beads (Raf-1 RBD, agarose) provided with the assay kit for 1 h at 4°C. The precipitates were washed thrice with Mg2+ lysis/washing buffer and eluted by boiling in 40 µl 2x SDS-PAGE sample buffer. The proteins were separated on a 12% SDS-polyacrylamide gel and then immunoblotted with a pan-Ras antibody (supplied with the kit). To normalize the amount of GTP-bound Ras to total amount of Ras, equal volumes of cell lysate were also subjected to Western blot analysis using the pan-Ras antibody.
ChIP Assay
To detect the in vivo association of nuclear proteins with the cyclin D1 and VEGF promoters, ChIP assays were conducted using the protocol described by Upstate Biotechnology with some modifications. In brief, PMKs (1 × 107) were serum starved for 24 h and then stimulated for 30 min with PGE2 and protein–DNA complexes were fixed with 1% formaldehyde solution for 10 min at 37°C. The cells were washed twice with ice-cold PBS containing protease inhibitors and scraped into a conical tube, centrifuged for 5 min at 2000 rpm, lysed in 200 µl of SDS buffer (1% SDS, 10 mM EDTA and 50 mM Tris-HCl, pH 8.1), and placed on ice for 10 min. The cell lysate was then sonicated to generate 200- to 1,000-bp DNA fragments. After centrifugation, the cleared supernatant was diluted 10-fold with ChIP buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, 16.7 mM NaCl and protease inhibitors, pH 8.0). Chromatin solutions were incubated with 1.5 µg of CREB or c-Jun (Cell Signaling) or p65, c-Fos (Santa Cruz Biotecnology) antibody at 4°C and immune complexes were mixed with 60 µl of salmon sperm DNA/protein A/G agarose slurry (Upstate Biotechnology) for 1 h. Normal mouse IgG (Santa Cruz Biotecnology) was used instead of specific antibody in the negative control. The protein A/G agarose-antibody-chromatin complex was pelleted by centrifugation at 2000 rpm for 1 min at 4°C. The pellet was extensively washed and eluted by resuspension in fresh elution buffer (1% SDS and 50 mM NaHCO3). Twenty microliters of 5 M NaCl was added to the supernatant, and the mixture was incubated at 65°C for 4 h to reverse histone-DNA cross-linking. The DNA fragments were purified using the phenol/chloroform extraction method and dissolved in 20 µl water. Each sample (5 µl) was used as a template for PCR amplification. Cyclin D1 oligonucleotide sequence for PCR primers were 5'-CCTCAACGAAGCCAATCAAG-3' and 5'-CAGTATCCCCCTCCTCCACT-3'. This primer set encompasses the cyclin D1 promoter segment that includes the CRE and TRE binding sites. Other cyclin D1 oligonucleotide sequences for PCR primers were 5'-CCGGCTTTGATCTCTGCTTA-3' and 5'-GCTGTACTGCCGGTCTCC-3'. This primer set encompasses the cyclin D1 promoter segment that includes the AP-1 binding site. VEGF oligonucleotide sequences for PCR primers were 5'-TGTGTCAATGTGAGTGCGTGC-3' and 5'-GCGGTGGAAGAAAAAGAGGAAATC-3'. These primers amplify the VEGF promoter segment that includes the CRE and TRE binding site. Other VEGF oligonucleotide sequences for PCR primers were 5'-GCAGCTGGCCTACCTACCTT-3' and 5'-ACTGAGAACGGGAAGTGGAG-3'. This primer set encompasses the VEGF promoter surrounding the AP-1 binding site. PCR mixtures were amplified for 1 cycle at 94°C for 5 min followed by 35 cycles 94°C 30 s, 60°C for 30 s, and 72°C for 45 s and then subjected to final elongation at 72°C for 10 min. PCR products were run on 2% agarose gel and analyzed by ethidium bromide staining. All ChIP assays were performed at least three times with similar results.
Statiscal Analysis
Data are shown as the mean ± SD. Statistical differences between means were determined by one-way ANOVA, using SPSS 10 (SPSS Mac V.10, SPSS, Chicago, IL, USA).
Supplementary Material
Acknowledgments
This work was supported by grants from NCI CA100140, CA105345 (SMF), CA16672 and NIEHS ES07784
References
- 1.Fischer SM. Eicosanoids and tumor promotion. In: Muhktar H, editor. Skin Cancer: Mechanisms and Human Relevance. Boca Raton, FL: CRC Press; 1995. pp. 129–144. [Google Scholar]
- 2.Fischer SM. Prostaglandins and cancer. Front Biosci. 1997;2:d482–d500. doi: 10.2741/a207. [DOI] [PubMed] [Google Scholar]
- 3.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 4.Flower RJ. Prostaglandins, bioassay and inflammation. Br J Pharmacol. 2006;147 Suppl 1:S182–S192. doi: 10.1038/sj.bjp.0706506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 6.Fischer SM. Is cyclooxygenase-2 important in skin carcinogenesis? J Environ Pathol Toxicol Oncol. 2002;21:183–191. [PubMed] [Google Scholar]
- 7.Fischer SM, Furstenberger G, Marks F, Slaga TJ. Events associated with mouse skin tumor promotion with respect to arachidonic acid metabolism: A comparison between Sencar and NMRI mice. Cancer Research. 1987;47:3174–3179. [PubMed] [Google Scholar]
- 8.Furstenberger GMF. The role of eicosanoids in normal, hyperplastic and neoplastic skin. Boca Raton, FL: CRC Press; 1990. pp. 107–124. [Google Scholar]
- 9.Fischer SM, Gleason GL, Bohrman JL, Slaga TJ. Prostaglandin enhancement of skin tumor initiation and promotion. Adv Prostaglandin Thromboxane Res. 1980;6:517–522. [PubMed] [Google Scholar]
- 10.Ruzicka T, Walter JF, Printz MP. Changes in arachidonic acid metabolism in UV-irradiated hairless mouse skin. J Invest Dermatol. 1983;81:300–303. doi: 10.1111/1523-1747.ep12519287. [DOI] [PubMed] [Google Scholar]
- 11.Karmali RA. Eicosanoids in neoplasia. Prev Med. 1987;16:493–502. doi: 10.1016/0091-7435(87)90063-6. [DOI] [PubMed] [Google Scholar]
- 12.Aizu E, Yamamoto S, Nakadate T, Kato R. Differential effects of various skin tumor-promoting agents on prostaglandin E2 release from primary cultures of mouse epidermal cells. Eur J Pharmacol. 1990;182:19–28. doi: 10.1016/0014-2999(90)90489-s. [DOI] [PubMed] [Google Scholar]
- 13.Goldyne ME, Evans CB. 12-O-tetradecanoylphorbol-13-acetate and the induction of prostaglandin E2 generation by human keratinocytes: a re-evaluation. Carcinogenesis. 1994;15:141–143. doi: 10.1093/carcin/15.1.141. [DOI] [PubMed] [Google Scholar]
- 14.Furstenberger G, Marks F. Early prostaglandin E synthesis is an obligatory event in the induction of cell proliferation in mouse epidermis in vivo by the phorbol ester TPA. Biochem Biophys Res Commun. 1980;92:749–756. doi: 10.1016/0006-291x(80)90767-6. [DOI] [PubMed] [Google Scholar]
- 15.Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, Conti CJ. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25:231–240. [PubMed] [Google Scholar]
- 16.Tiano HF, Loftin CD, Akunda J, Lee CA, Spalding J, Sessoms A, Dunson DB, Rogan EG, Morham SG, Smart RC, Langenbach R. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–3401. [PubMed] [Google Scholar]
- 17.Rundhaug JE, Pavone A, Kim E, Fischer SM. The effect of cyclooxygenase-2 overexpression on skin carcinogenesis is context dependent. Mol Carcinog. 2007;46:981–992. doi: 10.1002/mc.20340. [DOI] [PubMed] [Google Scholar]
- 18.Rys-Sikora KE, Konger RL, Schoggins JW, Malaviya R, Pentland AP. Coordinate expression of secretory phospholipase A(2) and cyclooxygenase-2 in activated human keratinocytes. Am J Physiol Cell Physiol. 2000;278:C822–C833. doi: 10.1152/ajpcell.2000.278.4.C822. [DOI] [PubMed] [Google Scholar]
- 19.Furstenberger G, Marks F. Indomethacin inhibition of cell proliferation induced by the phorbolester TPA is reversed by prostaglandin E2 in mouse epidermis in vivo. Biochem Biophys Res Commun. 1978;84:1103–1111. doi: 10.1016/0006-291x(78)91697-2. [DOI] [PubMed] [Google Scholar]
- 20.Pentland AP, Needleman P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J Clin Invest. 1986;77:246–251. doi: 10.1172/JCI112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Lee JL, Kim A, Kopelovich L, Bickers DR, Athar M. Differential expression of E prostanoid receptors in murine and human non-melanoma skin cancer. J Invest Dermatol. 2005;125:818–825. doi: 10.1111/j.0022-202X.2005.23829.x. [DOI] [PubMed] [Google Scholar]
- 23.Sung YM, He G, Fischer SM. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res. 2005;65:9304–9311. doi: 10.1158/0008-5472.CAN-05-1015. [DOI] [PubMed] [Google Scholar]
- 24.Rundhaug JE, Mikulec C, Pavone A, Fischer SM. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 2007;46:692–698. doi: 10.1002/mc.20329. [DOI] [PubMed] [Google Scholar]
- 25.Donnini S, Finetti F, Solito R, Terzuoli E, Sacchetti A, Morbidelli L, Patrignani P, Ziche M. EP2 prostanoid receptor promotes squamous cell carcinoma growth through epidermal growth factor receptor transactivation and iNOS and ERK1/2 pathways. Faseb J. 2007;21:2418–2430. doi: 10.1096/fj.06-7581com. [DOI] [PubMed] [Google Scholar]
- 26.Geller AC, Annas GD. Epidemiology of melanoma and nonmelanoma skin cancer. Semin Oncol Nurs. 2003;19:2–11. doi: 10.1053/sonu.2003.50000. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 28.Chun KS, Akunda JK, Langenbach R. Cyclooxygenase-2 inhibits UVB-induced apoptosis in mouse skin by activating the prostaglandin E2 receptors, EP2 and EP4. Cancer Res. 2007;67:2015–2021. doi: 10.1158/0008-5472.CAN-06-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65:1822–1829. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- 31.O'Toole A, Moule SK, Lockyer PJ, Halestrap AP. Tumour necrosis factor-alpha activation of protein kinase B in WEHI-164 cells is accompanied by increased phosphorylation of Ser473, but not Thr308. Biochem J. 2001;359:119–127. doi: 10.1042/0264-6021:3590119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 33.Ansari KM, Sung YM, He G, Fischer SM. Prostaglandin receptor EP2 is responsible for cyclooxygenase-2 induction by prostaglandin E2 in mouse skin. Carcinogenesis. 2007 doi: 10.1093/carcin/bgm011. [DOI] [PubMed] [Google Scholar]
- 34.Fiedler MA, Wernke-Dollries K, Stark JM. Inhibition of TNF-alpha-induced NF-kappaB activation and IL-8 release in A549 cells with the proteasome inhibitor MG-132. Am J Respir Cell Mol Biol. 1998;19:259–268. doi: 10.1165/ajrcmb.19.2.3149. [DOI] [PubMed] [Google Scholar]
- 35.Maldve RE, Kim Y, Muga SJ, Fischer SM. Prostaglandin E(2) regulation of cyclooxygenase expression in keratinocytes is mediated via cyclic nucleotide-linked prostaglandin receptors. J Lipid Res. 2000;41:873–881. [PubMed] [Google Scholar]
- 36.Bol DK, Rowley RB, Ho CP, Pilz B, Dell J, Swerdel M, Kiguchi K, Muga S, Klein R, Fischer SM. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 2002;62:2516–2521. [PubMed] [Google Scholar]
- 37.Thompson EJ, Gupta A, Vielhauer GA, Regan JW, Bowden GT. The growth of malignant keratinocytes depends on signaling through the PGE(2) receptor EP1. Neoplasia. 2001;3:402–410. doi: 10.1038/sj.neo.7900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casanova ML, Larcher F, Casanova B, Murillas R, Fernandez-Acenero MJ, Villanueva C, Martinez-Palacio J, Ullrich A, Conti CJ, Jorcano JL. A critical role for ras-mediated, epidermal growth factor receptor-dependent angiogenesis in mouse skin carcinogenesis. Cancer Res. 2002;62:3402–3407. [PubMed] [Google Scholar]
- 39.Chen N, Ma WY, She QB, Wu E, Liu G, Bode AM, Dong Z. Transactivation of the epidermal growth factor receptor is involved in 12-O-tetradecanoylphorbol-13-acetate-induced signal transduction. J Biol Chem. 2001;276:46722–46728. doi: 10.1074/jbc.M107156200. [DOI] [PubMed] [Google Scholar]
- 40.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 41.Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. 2003;24:1515–1524. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 42.Bourcier C, Jacquel A, Hess J, Peyrottes I, Angel P, Hofman P, Auberger P, Pouyssegur J, Pages G. p44 mitogen-activated protein kinase (extracellular signal-regulated kinase 1)-dependent signaling contributes to epithelial skin carcinogenesis. Cancer Res. 2006;66:2700–2707. doi: 10.1158/0008-5472.CAN-05-3129. [DOI] [PubMed] [Google Scholar]
- 43.Engelberg D. Stress-activated protein kinases-tumor suppressors or tumor initiators? Semin Cancer Biol. 2004;14:271–282. doi: 10.1016/j.semcancer.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 45.Bachelor MA, Bowden GT. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin Cancer Biol. 2004;14:131–138. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Bachelor MA, Cooper SJ, Sikorski ET, Bowden GT. Inhibition of p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase decreases UVB-induced activator protein-1 and cyclooxygenase-2 in a SKH-1 hairless mouse model. Mol Cancer Res. 2005;3:90–99. doi: 10.1158/1541-7786.MCR-04-0065. [DOI] [PubMed] [Google Scholar]
- 47.Budunova IV, Perez P, Vaden VR, Spiegelman VS, Slaga TJ, Jorcano JL. Increased expression of p50-NF-kappaB and constitutive activation of NF-kappaB transcription factors during mouse skin carcinogenesis. Oncogene. 1999;18:7423–7431. doi: 10.1038/sj.onc.1203104. [DOI] [PubMed] [Google Scholar]
- 48.Suzukawa K, Weber TJ, Colburn NH. AP-1, NF-kappa-B, and ERK activation thresholds for promotion of neoplastic transformation in the mouse epidermal JB6 model. Environ Health Perspect. 2002;110:865–870. doi: 10.1289/ehp.02110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujino H, Salvi S, Regan JW. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol Pharmacol. 2005;68:251–259. doi: 10.1124/mol.105.011833. [DOI] [PubMed] [Google Scholar]
- 50.Pozzi A, Yan X, Macias-Perez I, Wei S, Hata AN, Breyer RM, Morrow JD, Capdevila JH. Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. J Biol Chem. 2004;279:29797–29804. doi: 10.1074/jbc.M313989200. [DOI] [PubMed] [Google Scholar]
- 51.Xian W, Rosenberg MP, DiGiovanni J. Activation of erbB2 and c-src in phorbol ester-treated mouse epidermis: possible role in mouse skin tumor promotion. Oncogene. 1997;14:1435–1444. doi: 10.1038/sj.onc.1200980. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto T, Jiang J, Kiguchi K, Carbajal S, Rho O, Gimenez-Conti I, Beltran L, DiGiovanni J. Overexpression of a constitutively active form of c-src in skin epidermis increases sensitivity to tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Mol Carcinog. 2002;33:146–155. doi: 10.1002/mc.10030. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto T, Jiang J, Kiguchi K, Ruffino L, Carbajal S, Beltran L, Bol DK, Rosenberg MP, DiGiovanni J. Targeted expression of c-Src in epidermal basal cells leads to enhanced skin tumor promotion, malignant progression, and metastasis. Cancer Res. 2003;63:4819–4828. [PubMed] [Google Scholar]
- 54.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 55.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 56.Hsu TC, Young MR, Cmarik J, Colburn NH. Activator protein 1 (AP-1)- and nuclear factor kappaB (NF-kappaB)-dependent transcriptional events in carcinogenesis. Free Radic Biol Med. 2000;28:1338–1348. doi: 10.1016/s0891-5849(00)00220-3. [DOI] [PubMed] [Google Scholar]
- 57.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 58.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 60.Robles AI, Rodriguez-Puebla ML, Glick AB, Trempus C, Hansen L, Sicinski P, Tennant RW, Weinberg RA, Yuspa SH, Conti CJ. Reduced skin tumor development in cyclin D1-deficient mice highlights the oncogenic ras pathway in vivo. Genes Dev. 1998;12:2469–2474. doi: 10.1101/gad.12.16.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larcher F, Murillas R, Bolontrade M, Conti CJ, Jorcano JL. VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene. 1998;17:303–311. doi: 10.1038/sj.onc.1201928. [DOI] [PubMed] [Google Scholar]
- 62.Desdouets C, Matesic G, Molina CA, Foulkes NS, Sassone-Corsi P, Brechot C, Sobczak-Thepot J. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Filmus J, Robles AI, Shi W, Wong MJ, Colombo LL, Conti CJ. Induction of cyclin D1 overexpression by activated ras. Oncogene. 1994;9:3627–3633. [PubMed] [Google Scholar]
- 64.Segrelles C, Ruiz S, Santos M, Martinez-Palacio J, Lara MF, Paramio JM. Akt mediates an angiogenic switch in transformed keratinocytes. Carcinogenesis. 2004;25:1137–1147. doi: 10.1093/carcin/bgh132. [DOI] [PubMed] [Google Scholar]
- 65.Bolontrade MF, Stern MC, Binder RL, Zenklusen JC, Gimenez-Conti IB, Conti CJ. Angiogenesis is an early event in the development of chemically induced skin tumors. Carcinogenesis. 1998;19:2107–2113. doi: 10.1093/carcin/19.12.2107. [DOI] [PubMed] [Google Scholar]
- 66.Sung YM, He G, Hwang DH, Fischer SM. Overexpression of the prostaglandin E2 receptor EP2 results in enhanced skin tumor development. Oncogene. 2006;25:5507–5516. doi: 10.1038/sj.onc.1209538. [DOI] [PubMed] [Google Scholar]
- 67.Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978;15:801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- 68.Shao J, Evers BM, Sheng H. Prostaglandin E2 synergistically enhances receptor tyrosine kinase-dependent signaling system in colon cancer cells. J Biol Chem. 2004;279:14287–14293. doi: 10.1074/jbc.M313276200. [DOI] [PubMed] [Google Scholar]
- 69.Yuspa SH, Harris CC. Altered differentiation of mouse epidermal cells treated with retinyl acetate in vitro. Exp Cell Res. 1974;86:95–105. doi: 10.1016/0014-4827(74)90653-3. [DOI] [PubMed] [Google Scholar]
- 70.Kim Y, Fischer SM. Transcriptional regulation of cyclooxygenase-2 in mouse skin carcinoma cells. Regulatory role of CCAAT/enhancer-binding proteins in the differential expression of cyclooxygenase-2 in normal and neoplastic tissues. J Biol Chem. 1998;273:27686–27694. doi: 10.1074/jbc.273.42.27686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.