Figure 6.
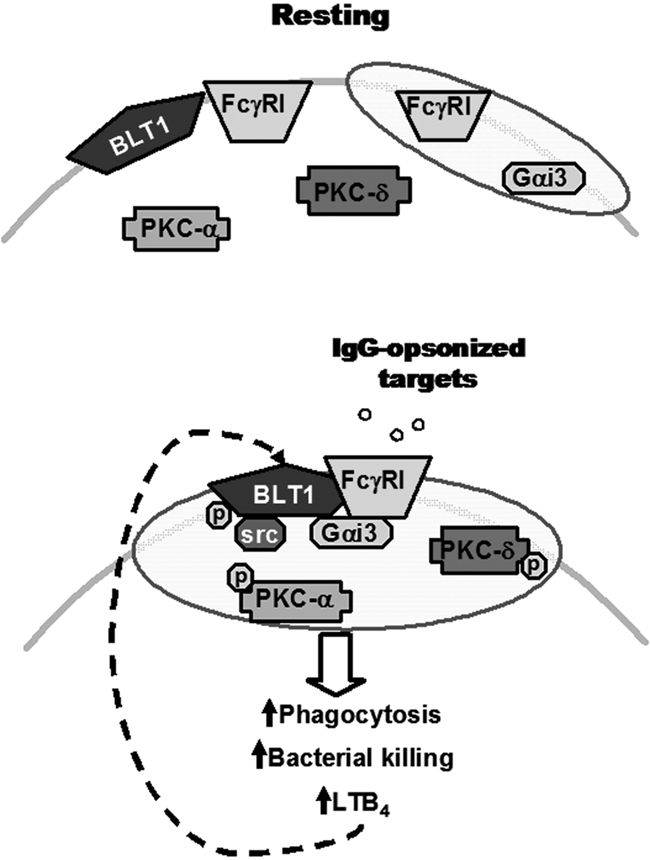
Proposed model for the IgG-induced FcγRI/BLT1/Gαi3/Src complex in LRs that mediates LTB4 enhancement of AM antimicrobial functions and signaling. (Top) In resting cells, BLT1, PKC-α, PKC-δ, and a proportion of FcγRI are located in non-LR domains, whereas Gαi3 and a proportion of FcγRI are constitutively present in LRs (indicated as shaded ovals). (Bottom) Upon FcγRI engagement, Src kinase phosphorylates BLT1, which promotes BLT1 redistribution to LRs as well as the formation of a complex within LRs consisting of this GPCR, its preferentially coupled G protein Gαi, FcγRI, and Src itself. Addition of exogenous LTB4 causes LRs to become further enriched in PKC isoforms, which are required for optimal ingestion of IgG-coated targets. Thus, LRs serve as platforms within which phosphorylated BLT1 interacts with FcγRI and downstream signaling molecules to mediate the augmentation by LTB4 of AM antimicrobial functions, explaining the LR dependence of LTB4/BLT1 actions. Signals from FcγRI also enhance LTB4 generation, which amplifies AM function in an autocrine and paracrine manner. The relative size of the trapezoids represents the relative amount of FcγRI in a given membrane domain. Phosphorylation is indicated by “p.”
