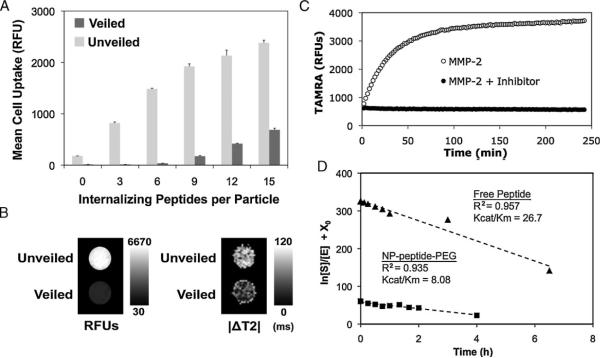
Figure 2.
Optimization and characterization of nanoparticle veiling, activation, and internalization. A) A library of nanoparticles with removable polymer coatings and a varying density of internalization ligands was screened for relative uptake by HT-1080 cancer cells before (veiled, dark gray) and after (unveiled, light gray) MMP cleavage. A density of 6 cell-internalizing peptides per particle demonstrated optimal veiling and internalization. Error bars are standard deviations from three separate experiments. B) Cells incubated with veiled and unveiled nanoparticles for 5 h were imaged by (left) a fluorescence scanner or (right) MRI, demonstrating the dual contrast properties of the nanoparticles and the correlated fluorescent and magnetic domain uptake of unveiled particles. C) MMP-mediated removal of polymer coatings relieves TAMRA-iron quenching interactions, thereby enabling remote monitoring of protease activation. D) The Kcat/Km for peptide-polymer NPs (squares) and free peptide (triangles) was determined to be 8.42 and 26.7 μM-1h-1, respectively, by measuring the cleavage of the substrate by MMP-2 over time. Polymer veiling and immobilization of the cleavable peptide substrate reduces its associated MMP-2 Kcat/Km within a practical range, 3.2 fold.