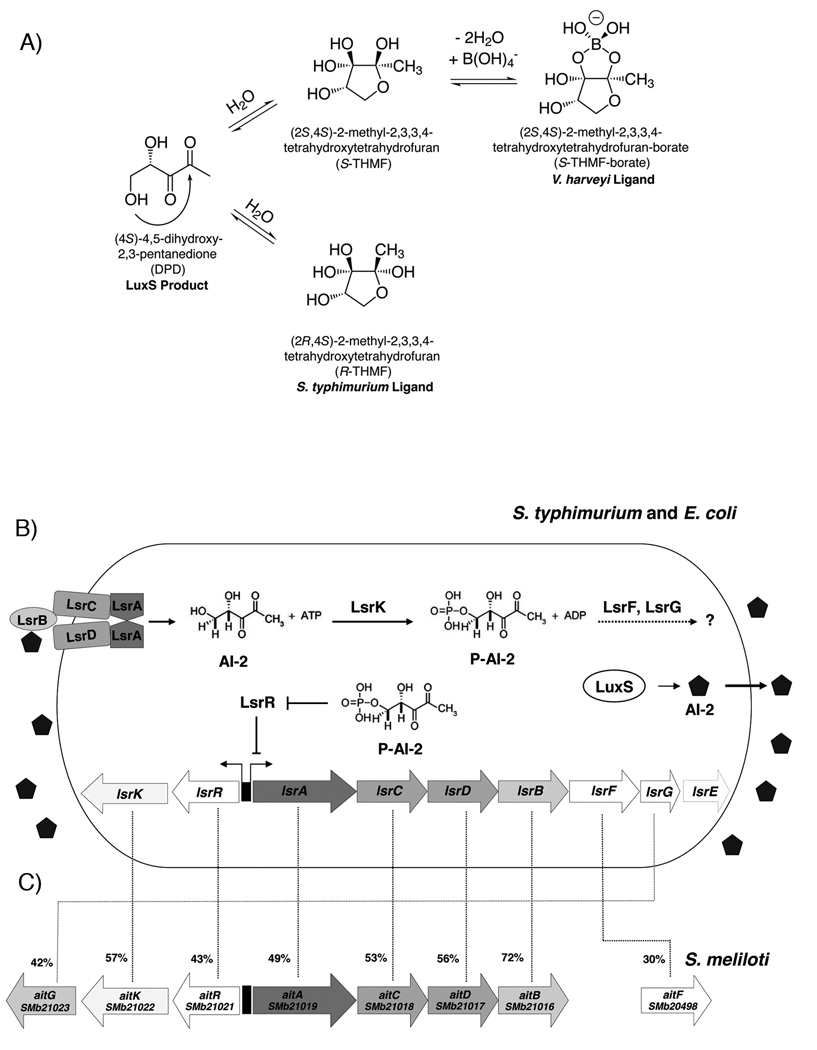
Fig. 1. The interconversion of DPD into the known AI-2 ligands and the AI-2-dependent internalization system.
A. Proposed equilibrium between the currently known forms of AI-2 and their common precursor, DPD. The V. harveyi and S. typhimurium ligands have previously been shown to interconvert in solution.
B. S. typhimurium and E. coli Lsr-mediated transport and processing of AI-2. In S. typhimurium and E. coli, AI-2 is produced within the cell by LuxS and is secreted to the medium. As the concentration of extracellular AI-2 increases, AI-2 binds to the periplasmic binding protein LsrB and is internalized by the Lsr system, an ABC-type transport system. Once in the cytoplasm, AI-2 is phosphorylated (P-AI-2) by LsrK. P-AI-2 binds to the repressor of the lsr operon, LsrR, inactivating LsrR, relieving repression, and inducing transcription of lsr. This causes a rapid increase in the production of the Lsr transporter and, consequently removal of AI-2 from the environment. P-AI-2 is further processed by a mechanism not fully understood involving LsrG and LsrF. The lsrE gene is also present in the operon of S. typhimurium but not in E. coli and its function is not known.
C. The lsr orthologues in S. meliloti operon. S. meliloti has orthologs to all the genes of the lsr operon except lsrE. We named the S. meliloti lsr-like operon ait (for autoinducer transporter). The percent identity to the Lsr proteins from S. typhimurium is shown.