Figure 2. Lnx-l binds and ubiquitinates Boz but not Gsc.
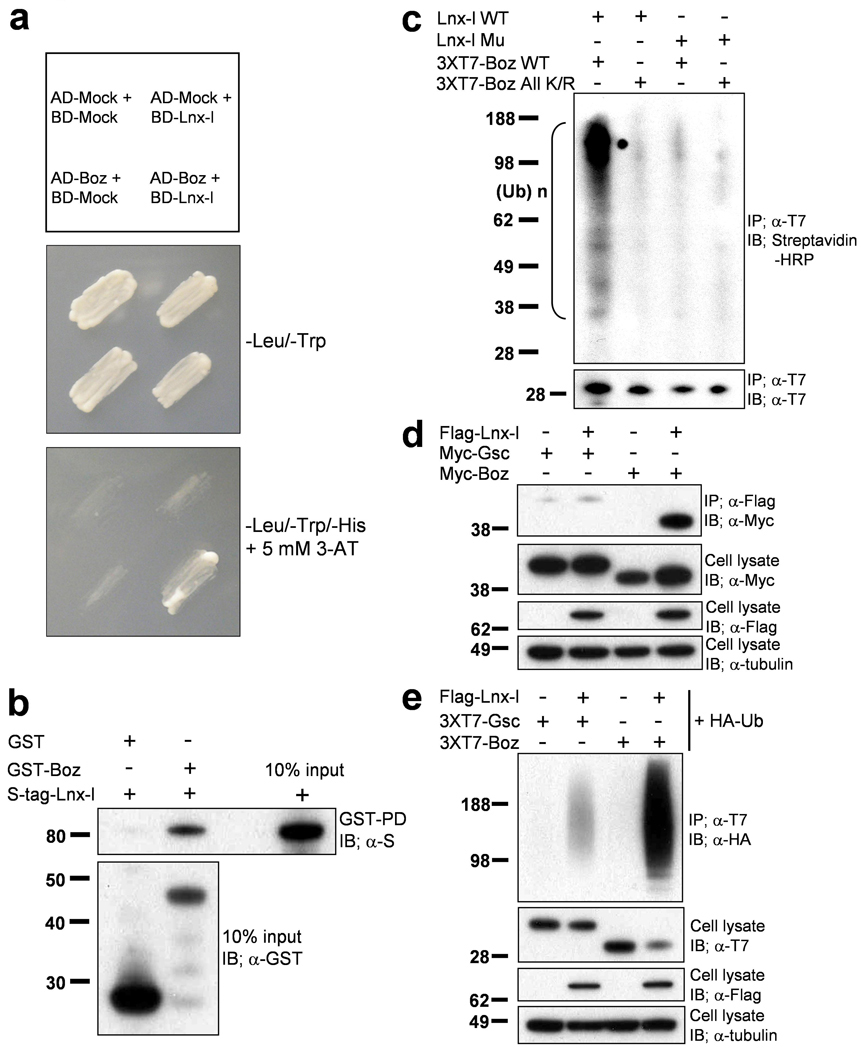
a, Lnx-l-Boz interaction in yeast. Yeast cells were cotransformed with the indicated fusion constructs (upper panel; see Methods). The transformants were plated on –Leu-Trp drop-out plates (middle panel) for 3 days. Individual transformants were transferred to –Leu-Trp-His drop-out plates containing 5 mM 3-AT, and incubated for 4 days (bottom panel). Only Boz-Lnx-l co-transformants showed substantial growth, indicating interaction between the two proteins. b, Direct interaction between Lnx-l and Boz was confirmed by the GST-pull down assay with proteins purified from bacteria (see Methods); GST alone could not pull-down S-tagged Lnx-l. c, In vitro ubiquitination assay was performed as described in Methods. Wt Boz mixed with extracts containing Wt Lnx-l yielded abundant ubiquitinated products, while RING mutant Lnx-l did not ubiquitinate Wt Boz, and Wt Lnx-l did not ubiquitinate Boz All K/R. d, Lnx-l interacts with Boz but not with Gsc. Flag-tagged Lnx-l was contransfected into 293T cells with 6xMyc-tagged Gsc or Boz. After 48 h, IP and IB were performed with the indicated antibodies. e, Lnx-l ubiquitinates Boz but not Gsc. Flag-tagged Lnx-l was contransfected with 3xT7 tagged Gsc or Boz. The yield of polyubiquitinated Gsc is very low compared to that of Boz (see also Fig. 3, below).. α-tubulin was used as loading control.
