Figure 3. Lnx-l-mediated polyubiquitination destabilizes Boz.
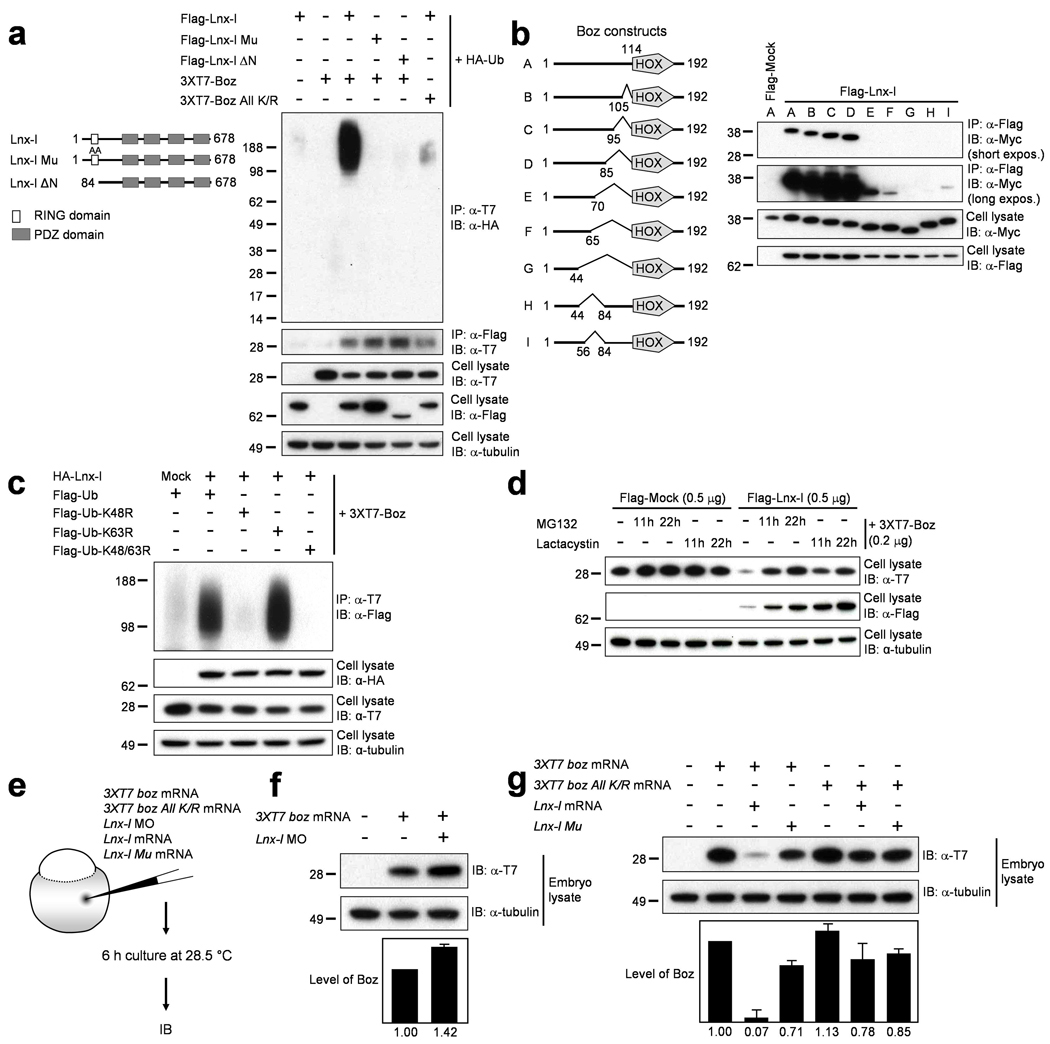
a,Flag-tagged Lnx-l, 3xT7-Boz, and HA-ubiquitin were cotransfected into 293T cells as indicated. Lnx-l constructs are shown on the left. Only Wt Boz is significantly polyubiquitinated by Wt Lnx-l. Molecular size markers are indicated at the left. b, Schematic diagrams of Boz deletion constructs are shown at the left. 6xMyc epitope-tagged Boz constructs were cotransfected with Flag-Lnx-l. Upon IP with anti-Flag antibody, the coprecipitates were subjected to IB with anti-Myc antibody. Short and long exposures are shown to illustrate the range of interaction strength between Lnx-l and Boz. c, HA-tagged Lnx-l and 3xT7-tagged Boz were cotransfected with Flag-tagged Wt or mutant ubiquitin. The K48R ubiquitin mutant could not support Boz polyubiquitination. d, Transfected cells were incubated with MG132 or lactacystin, each at 10 µmolar, for the indicated times. Destabilization of Boz by Lnx-l cotransfection was inhibited by MG132 or lactacystin. Lnx-l itself was stabilized in a time-dependent manner by the proteasome inhibitors. e, Schematic diagram of experimental design in (f) and (g). f, 20 pg of 3xT7 boz mRNA was injected with or without 2.5 ng of lnx-l UTR MO. Depletion of endogenous Lnx-l increased Boz levels. g, 20 pg 3xT7 boz or 3xT7 boz All K/R mRNA was injected with 50 pg lnx-l or 50 pg lnx-l Mu mRNA. Wt Lnx-l strongly destabilizes Wt Boz. Error bars in f and g were obtained from two repeats each of two independent experiments. α-tubulin was used as loading control.
