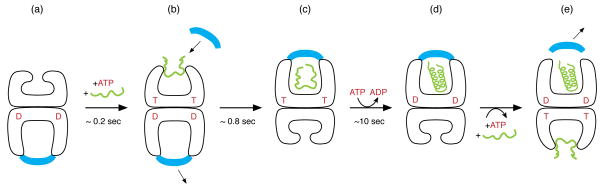
Fig. 1. Chaperonin reaction cycle.
An asymmetric GroEL-GroES-ADP complex (a) is the normal acceptor state for ATP (red; also indicated as T) and non-native polypeptide (green), binding them (b) in the open ring opposite the one bound by GroES (blue) and ADP (red D). ATP binding produces small rigid body apical domain movements in the bound ring (b), enabling GroES binding, attended by large rigid body movements that produce the stable folding-active cis complex end-state (c). This folding-active state is the longest-lived state of the reaction cycle, ~10 sec, followed by ATP hydrolysis (c→d), which then gates the entry of ATP and polypeptide into the opposite trans ring, rapidly discharging the cis ligands (e) and initiating a new folding active cycle on the ATP/polypeptide-bound ring.