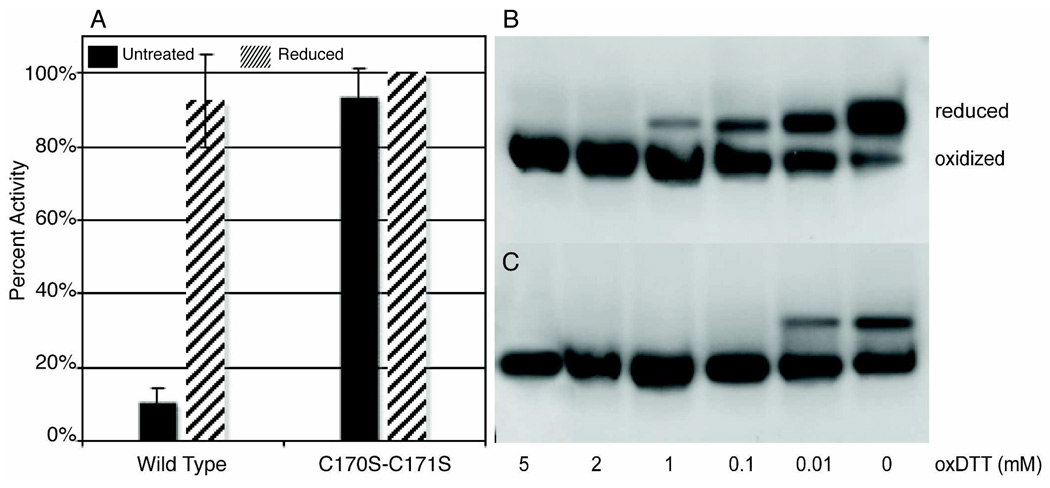
Figure 2. PRL-1-C170S-C171S.
A. The activity of PRL-1-C170S-C171S was measured as described in Figure 1. The activity of PRL-1-C170S-C171S is not modulated by the addition of DTT and is statistically identical under both non-reducing and reducing conditions. There is also no statistically significant difference in activity between the reduced wild type and PRL-1-C170S-C171S mutant, indicating that this mutant is primarily in the reduced state even in the absence of thiol modulating agents. B. PRL-1-C170S-C171S was run on SDS-PAGE under various oxidizing conditions (0–5 mM oxidized DTT). Under non-reducing conditions (lane 6), C170S-C171S runs as two bands. In contrast to wild type, however, C170S-C171S is primarily reduced as indicated by the much greater intensity of the upper reduced band. The small amount of oxidized protein is reduced upon addition of 3 mM DTT (data not shown). Complete oxidation of PRL-1-C170-171S occurs near 2 mM oxidized DTT. C. PRL-1-WT was run on SDS-PAGE under the same conditions as in B. Complete oxidation of wild type occurs near 0.1 mM oxidized DTT. In both B and C panels, samples were prepared as in figure 1.