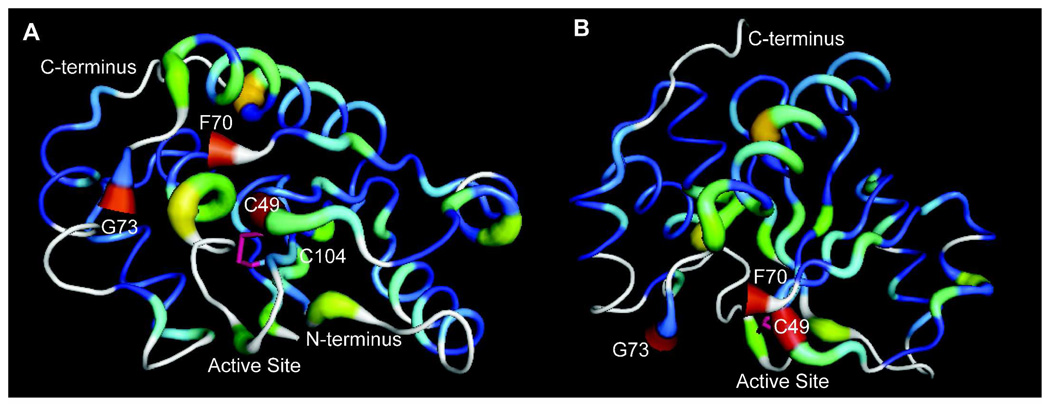
Figure 5. Comparisons of PRL-1-WT and C170S-C171S.
A. Differences in NMR chemical shifts between PRL-1-WT and PRL-1-C170-C171S were mapped to the crystal structure (as in figure 4) and displayed with the active site in front. The disulfide bound (pink bond) C104 and C49 are labeled. F70 and G73 are labeled to identify the break in the crystal structure. B. As in A, but with the active site rotated downward by approximately 90 degress to reveal the large number of chemical shift deviations in the majority of the protein structure, especially in the central β-strand. Both figures were generated using Pymol.