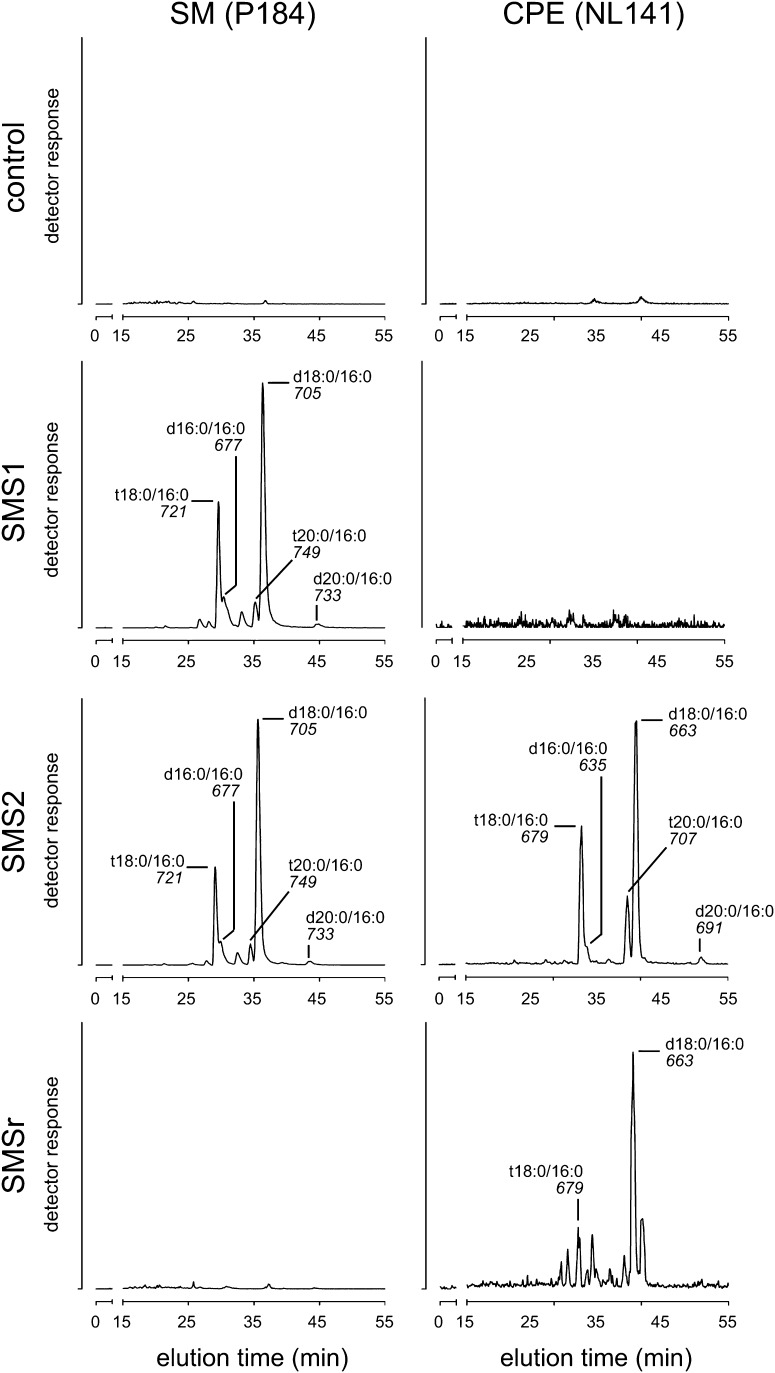
Fig. 3.
SMS2 displays dual activity as SM and CPE synthase in vivo. The substrate specificities of human SMS1, SMS2, and SMSr were investigated by heterologous expression in yeast. The yeast strain 4Δ.CerS5 used in this experiment provides ceramides containing a C16 fatty acid as substrates for the biosynthesis of SM or CPE (15). Several molecular species of SM and CPE were detected in yeast cells expressing SMS2, while only SM or CPE species were detected in cells expressing SMS1 or SMSr, respectively. The ceramide backbone composition of the SM or CPE formed was similar and independent of the enzyme (SMS1, SMS2, or SMSr). SM species were detected by LC/MS as precursors of the fragment ion with m/z = +184 corresponding to phosphocholine (P184) and CPE species by a neutral loss of 141 Da corresponding to phosphoethanolamine (NL141). The nominal mass of the most intense ion and the ceramide backbone composition of the corresponding SM or CPE species are indicated for each peak. The sphingoid base composition (d18:0, sphinganine; t18:0, phytosphinganine, as well as C16 and C20 derivatives thereof) was inferred assuming that the ceramides in the yeast strain 4Δ.CerS5 contain almost exclusively palmitic acid (16:0) (15).