Fig. 4.
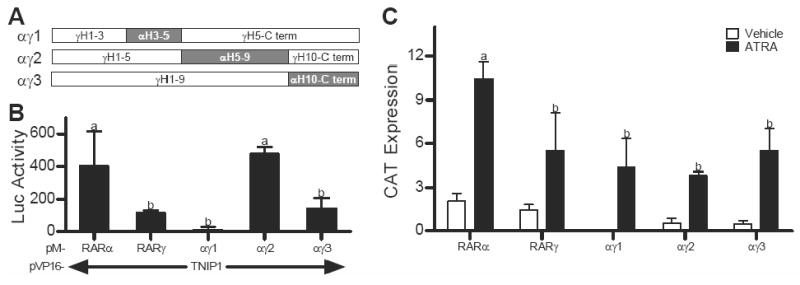
Replacement of helices 5-9 of RARγ ligand binding domain (LBD) with those of RARα increases RARγ-TNIP1 interaction. (A) Schematic of LBD helices in RARα/RARγ chimera constructs with RARγ as open and RARα replacements as shaded rectangles, C term: carboxyl terminus. (B) M2H assays in ATRA treated Cos-7 cells showing interaction of RARα, RARγ or αγ chimeras with TNIP1 presented as difference in reporter activation between TNIP1 constructs and empty vector control. (C) Activation of TRE-tk-CAT reporter by RARα, RARγ, or RARα/RARγ chimeras in Cos-7 cells presented as difference in reporter activation between receptor constructs and empty vector control. RXRα was cotransfected with all RAR constructs. In (B) and (C) bars with different letter indicate significantly different values.
