Abstract
Annual ryegrass (Lolium multiflorum Lam.) was grown in paddy soil in pots under different phosphorus (P) fertilizer treatments to investigate changes of P fractions and microbial community of the soil. The treatments included Kunyang phosphate rock (KPR) applications at 50 mg P/kg (KPR50) and 250 mg P/kg (KPR250), mono-calcium phosphate (MCP) application at 50 mg P/kg (MCP50), and the control without P application. The results showed that KPR50, KPR250, and MCP50 applications significantly increased the dry weight of the ryegrass by 13%, 38%, and 55%, and increased P uptake by 19%, 135%, and 324%, respectively. Compared with MCP50, the relative effectiveness of KPR50 and KPR250 treatments in ryegrass production was about 23% and 68%, respectively. After one season of ryegrass growth, the KPR50, KPR250, and MCP50 applications increased soil-available P by 13.4%, 26.8%, and 55.2%, respectively. More than 80% of the applied KPR-P remained as HCl-P fraction in the soil. Phospholipid fatty acid (PLFA) analysis showed that the total and bacterial PLFAs were significantly higher in the soils with KPR250 and MCP50 treatments compared with KPR50 and control. The latter had no significant difference in the total or bacterial PLFAs. The KPR50, KPR250, and MCP50 treatments increased fungal PLFA by 69%, 103%, and 69%, respectively. Both the principal component analysis and the cluster analysis of the PLFA data suggest that P treatments altered the microbial community composition of the soils, and that P availability might be an important contributor to the changes in the microbial community structure during the ryegrass growth in the paddy soils.
Keywords: Phosphorus fractionation, Phospholipid fatty acid (PLFA), Ryegrass, Phosphate rock
INTRODUCTION
Phosphorus (P) is considered the prime limiting factor on plant growth in many areas, because it is the least mobile and available essential nutrient in soil (Hinsinger, 2001). P fertilizer application is needed to sustain optimum plant production and quality (Zapata and Zaharah, 2002). The main objective of P management is to prevent P deficiency rather than to alleviate P-deficiency symptoms. If soil P supply is low, management must be focused on the buildup and maintenance of adequate soil-available P levels to ensure that P supply does not limit crop growth and N-use efficiency (Fairhurst and Witt, 2002). In the past, numerous studies were focused on possible substitution of phosphate rock (PR) for water-soluble P fertilizers mainly based on agronomic and economic considerations. Results have shown that PR can be as effective as superphosphate in increasing plant yield and improving soil P status in many tropic and subtropical areas (Chien et al., 1980; Bolan et al., 1990). PR applied to upland crops is more effective for rice in rice-upland crop rotation than for that in rice-rice system. It was suggested that the PRs be applied only to the upland crops so that the flooded rice could utilize residual available P (Meng et al., 2006). Recently, eutrophication of surface water bodies caused by excessive P from agricultural soil surface run-off or leaching has drawn many researchers to find strategies to mitigate the P pollution problem. It has been suggested that the use of reactive PR not only can sustain crop productivity but also may minimize eutrophication problems because of lower availability of PR for algal growth (Hart et al., 2004; Shigaki et al., 2006). However, more work including field studies is needed to validate this supposition.
When a PR is applied to acid soil, dissolution of PR releases P to soil solution (Chien et al., 1980). Part of the released P can be absorbed directly by the plant roots, and part of the released P reacts with soil components such as Fe-Al-oxides to form reaction products with different availabilities, which can provide available P later through different release processes. Thus, both the reaction products and undissolved PR can provide available P to the plant (Chien, 1978; Chien and Menon, 1995). However, the detail of the transformation process of the PR-P in paddy soil is still not very clear.
It is known that plant rhizosphere process may enhance the dissolution of PR (Hoffland et al., 1989; Bolan et al., 1997), and that the soil microbial community-inhabitants can accelerate dissolution of PR by producing organic acids, phenolic compounds, protons, and siderophores (Drever and Vance, 1994). Several research studies have identified microbial groups that could solubilize P minerals and improve plant P nutrition (Duponnois et al., 2005). Numerous studies have been conducted on the impact of nitrogen fertilizer, manure, and different management practices on soil microbial community (Lovell et al., 1995; Murray et al., 2006; Toyota and Kuninaga, 2006). However, there are few studies conducted on the impact of P fertilizer, particularly PR application on the composition of soil microbial community (Rooney and Clipson, 2009).
The objective of this study was to investigate the changes of P fractions and microbial community of soil planted with annual ryegrass (Lolium multiflorum Lam.) under different P fertilizer applications.
MATERIALS AND METHODS
Soil samples
A surface paddy soil sample (0~15 cm in depth) of alluvial deposit was taken from a long term site-specific nutrient management trial in Jinhua City (29°01′ N, 119°37′ E), Zhejiang Province, China. The soil was acidic (pH 4.81), containing 278 g/kg sand, 562 g/kg silt, 160 g/kg clay, 255 mg/kg total P, 4.35 mg/kg Olsen-P, and 2.5 g/kg FeDCB. Soil pH was measured in deionized water at a soil:solution ratio of 1:1. Total P was digested with H2SO4-HClO4, available P was extracted with the method of Olsen et al. (1954), and total free iron oxide was extracted with the method of Mehra and Jackson (1960). The soil was air-dried and ground to pass through a 2-mm sieve before potting.
Phosphorus sources
Kunyang phosphate rock (KPR), collected from Kunyang, Yunnan Province, China, was ground to pass through a 0.149-mm sieve. The KPR (pH 7.0 at solid:water=1:5 (w/v)) contained 138.5 g/kg total P and 29.1 g/kg of 2% (w/v) citric acid extractable P. The minerals of the KPR were identified by standard X-ray diffraction (Phillips-PW1732 X-diffractometer using nickel filter and Cu radiator with intensity of scan at (2°)/min at 40 kV and 20 mA). The empirical formula of apatite in Kunyang phosphate rock (KPR), was Ca9.83Na0.12Mg0.05(PO4)5.50(CO3)0.50F2.20. Mono-calcium phosphate (MCP, Ca(H2PO4)2·H2O) of analytical grade was used as water-soluble P fertilizer.
Pot experiment
A pot experiment was conducted in the greenhouse of Zhejiang University (China) in 2008. Treatments included applications of KPR at 50 and 250 mg P/kg (KPR50 and KPR250), application of MCP at 50 mg P/kg (MCP50), and the control (without P application). All the pots received 100 mg N/kg with urea and 50 mg K/kg with KCl. Fertilizer-P and Fertilizer-K were incorporated in the soil at the beginning of the experiment (basal). Fertilizer-N was applied with 50% as basal and 50% top-dressed 30 d after seeding. The pots were arranged in a randomized complete block design with three replicates. Approximately 30 seeds of ryegrass were sown per pot. After one-week growth, the ryegrasses were thinned to 20 plants per pot. All pots were irrigated with deionized water to maintain 80% soil water-holding capacity during the entire experiment.
The ground ryegrass plants were cut 70 d after seeding when the plants were about 30 cm high. They were then oven-dried, weighed, and ground to pass through a 2-mm sieve. The concentration of P in the plants was determined after digestion with H2SO4-H2O2 mixture. Soil sample was taken from each pot after harvest. One portion of the soil sample was air-dried and ground to pass through a 0.148-mm sieve for P fractionation, and another portion of the soil sample was freeze-dried immediately at −50 °C, and then stored at −20 °C for microbial community analysis. The root material was removed from the sieved soil samples before lipid extraction.
Phosphorus fractionation
Soil P was sequentially fractionated following the method of Hedley et al.(1982). A 0.50 g soil sample (<0.148 mm) was shaken for 16 h with one HCO3 − saturated resin capsule (purchased from UNIBEST Company, USA) enclosed in a nylon mesh-bag and immersed in 30 ml deionized water. After recovering the resin capsule, it was then placed in 30 ml of 2.0 mol/L HCl for P desorption. After centrifugation of the soil-water suspension, the supernatant was discarded. The remaining soil was then successively extracted with 0.5 mol/L NaHCO3 (pH 8.5), 0.1 mol/L NaOH, and 1 mol/L HCl each for 16 h. The inorganic P (Pi) concentration of each extract was determined and referred to as resin-, NaHCO3-, NaOH-, and HCl-extractable Pi fractions. Organic P (Po) in the resin and 1 mol/L HCl was not determined due to its negligible amount. Total P concentrations of NaHCO3 and NaOH extracts were determined after acid ammonium persulfate digestion (EPA, 1971). Po concentration of these extracts was calculated as the difference between the total and inorganic P, referred to as NaHCO3- and NaOH-extractable Po fractions. The soil residue was digested with 5 ml of concentrate H2SO4 and 0.5 ml of HClO4. All P determinations were done in duplicates using a colorimetric method (Murphy and Riley, 1962).
Phospholipid fatty acid analysis
Lipid extraction and phospholipid fatty acid (PLFA) analysis were performed with the method of Bossio et al.(1998). A 3-g soil sample (equivalent dry weight) was extracted with a chloroform-methanol-citrate buffer mixture, and the phospholipids were separated from other lipids on a silica-bonded phase column (SPE-Si, Supelco, Poole, UK). The phospholipid fraction was subjected to mild alkaline hydrolysis for producing fatty acid methyl esters before analysis. c19:0 was used as the internal standard. Fatty acids were analyzed by Agilent 6890 gas chromatography with a flame ionization detector carried out by an MIDI Sherlocks microbial identification system (Version 4.5, MIDI, Newark, NJ, USA).
The fatty acid nomenclature used in this study included total number of carbon atoms:number of double bonds, followed by the position of the double bond (ω) from the methyl end of the molecule. Cis and trans geometries were indicated by the suffixes c and t, respectively. The prefixes a and i referred to anteiso- and iso-branching, respectively. 10Me indicated a methyl group on the tenth carbon atom from the carboxyl end of the molecule, position of hydroxyl (OH) groups was noted, and cy indicated cyclopropane fatty acids (Bossio et al., 2006). Each value was represented by mean of three replicates.
Bacterial PLFAs were represented by i15:0, a15:0, 15:0, i16:0, 16:1ω7c, i17:0, a17:0, cy17:0, 17:0, 18:1ω7c, and cy19:0ω8c (Bossio et al., 1998), fungal PLFAs were represented by 18:2ω6,9c and 18:3ω6c (6,9,12) (Myers et al., 2001; Vestal and White, 1989), and actinomycetic PLFAs were represented by 16:0 (10Me), 17:0 (10Me), 18:0 (10Me) (Turpeinen et al., 2004).
Statistical analysis
Statistical analysis was carried out with SPSS Version 13.0. Analysis of variance (ANOVA) was performed by using Fisher’s least significant difference comparison of means (LSD). Principal component analysis (PCA) and cluster analysis of the PLFA data were carried out with the correlation matrix and Ward’s method, respectively.
RESULTS
Yield response and phosphorus uptake of ryegrass
The soil was acidic (pH 4.81) with low P status (4.35 mg/kg Olsen-P), which might favor PR dissolution. The pot experiment showed that KPR50, KPR250, and MCP50 applications significantly increased ryegrass dry weight by 13%, 38%, and 55%, and increased P uptake by 19%, 135%, and 324%, respectively (Table 1). Compared with MCP50, the effectiveness of KPR50 and KPR250 treatments was about 73% and 89% in dry weight and 28% and 55% in P uptake, respectively.
Table 1.
Dry weight of ryegrass and uptake of P
| Treatment | Dry weight (g/pot) | P uptake (mg/pot) |
| Control | 0.80±0.02d | 0.86±0.09c |
| KPR50 | 0.90±0.02c | 1.02±0.10c |
| KPR250 | 1.10±0.01b | 2.02±0.20b |
| MCP50 | 1.24±0.01a | 3.65±0.48a |
Data are expressed as mean±SD of three replicates. Means with the same letter within the same column are not significantly different at 0.05 level from each other
Phosphorus fractions
After one season of ryegrass growth, KPR50, KPR250, and MCP50 applications increased soil available P (summation of resin-P, NaHCO3-Pi, and NaHCO3-Po) by 13.4%, 26.8%, and 55.2%, respectively. NaOH-Pi also significantly increased while soil NaOH-Po was relatively stable and less affected by P additions (Table 2). Soil HCl-P substantially increased after KPR application. The HCl-P is defined as Ca-associated P (Cross and Schlesinger, 1995), so the amounts of the undissolved apatite mineral of KPR in the soil can be estimated from the increases in the amounts of HCl-P in the KPR-treated soil over control soil (Tambunan et al., 1993). More than 80% of the applied KPR-P remained in the soil during one season of ryegrass growth. The MCP50 application did not show a significant increase of HCl-P or residual-P.
Table 2.
Soil P fractions (mg/kg) with different P fertilizations after ryegrass planting
| P treatment | Resin-P | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | HCl-P | Residual-P |
| Control | 10.62c | 5.02d | 20.17b | 26.04d | 67.03a | 34.95c | 83.59a |
| KPR50 | 13.16b | 5.60c | 21.81b | 27.38c | 66.60a | 79.15b | 82.19a |
| KPR250 | 14.42b | 7.04b | 23.85a | 29.77b | 67.59a | 230.58a | 86.87a |
| MCP50 | 20.76a | 10.27a | 24.50a | 33.58a | 66.89a | 35.29c | 84.27a |
Means with the same letter within the same column are not significantly different at 0.05 level from each other
Phospholipid fatty acid
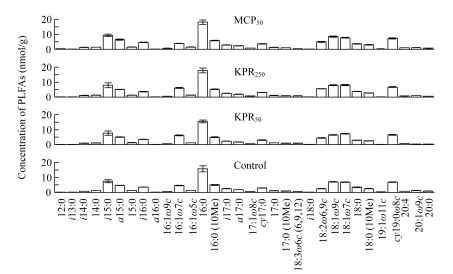
The predominant PLFAs in the soil were 16:0, i15:0, 18:1ω9c, 18:1ω7, cy19:0ω8c, and 16:0 (10Me) (Fig.1). P application significantly (P<0.05) increased PLFA 18:2ω6,9c. PLFA i15:0 was significantly (P<0.05) higher in soil with MCP50 application than in soil with KPR application and the control. KPR application produced more (P<0.05) PLFA 16:1ω7c compared with MCP50 and control.
Fig.1.
Concentrations of PLFAs in different P treatments
Table 3 shows PLFA concentrations of microbial groups in the soil, following the order of bacteria (PLFAbact)>actinomycete (PLFAacti)>fungi (PLFAfung). The total PLFA ranged from 90 to 106 nmol/g in the soil with different P treatments. The total PLFA and bacterial PLFA were significantly higher in the soil with KPR250 and MCP50 treatments compared with KPR50 and control while the latter two treatments were not statistically different from each other. The KPR50, KPR250, and MCP50 treatments increased fungal PLFAs by 69%, 103%, and 69%, respectively. P application also increased the ratio of PLFAfung/PLFAbact, which might be a good indicator for a shift in soil microbial community. PLFAacti did not change significantly with KPR applications while MCP50 significantly increased PLFAacti.
Table 3.
Distribution of PLFA profiles (nmol/g) in different P treated soils
| P treatment | PLFAbact | PLFAfung | PLFAfung/PLFAbact | PLFAacti | PLFAtotal |
| Control | 43.66c | 3.15c | 0.07 | 8.36b | 90.00b |
| KPR50 | 45.47bc | 5.41b | 0.12 | 8.43b | 91.31b |
| KPR250 | 47.61ab | 6.50a | 0.14 | 8.94ab | 101.19a |
| MCP50 | 50.83a | 5.39b | 0.11 | 9.90a | 106.00a |
PLFAbact, PLFAfung, PLFAacti, and PLFAtotal are bacterial, fungal, actinomycetic, and total PLFAs, respectively; Means with the same letters within the same column are not significantly different at 0.05 level from each other; n=6
Changes of soil microbial community
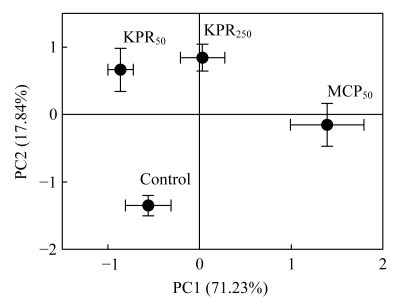
The analysis of principal components (PCs) of the PLFA data indicated that different P treatments were significantly discriminated by their PLFA profile (Fig.2). The variations of the first two principal components, PC1 and PC2, were 71.23% and 17.84%, respectively. MCP50 significantly discriminated the other treatments on PC1. The control had the lowest ordinate scores on PC2, and it was clearly discriminated from the other treatments.
Fig.2.
Principal component analysis of the PLFA profile in different P treated soils
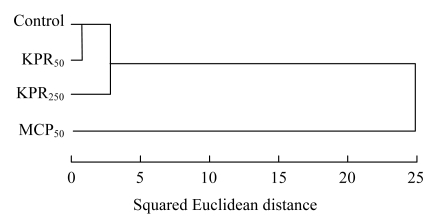
Cluster analysis of the PLFA data was also performed by using Ward’s method (Fig.3). The results show that the microbial community structure in the four treatments could be classified into two large clusters: (1) Control, KPR50, and KPR250 and (2) MCP50. The first cluster could be subdivided into two clusters: (1) Control and KPR50 and (2) KPR250.
Fig.3.
Cluster analysis of PLFA profile in different P treated soils
DISCUSSION
The sequential chemical extraction method developed by Hedley et al.(1982) has been widely used to chemically fractionate the continuum of soil P. It is generally assumed that P availability to plants decrease with increasing strength of the chemicals used in the fractionation procedure. Measurable P fractions such as resin- and bicarbonate-extractable P are thought to be labile-P that contributes most to plant available-P, while hydroxide- and acid-extractable P fractions are thought to be P forms of moderate or low availability to plants (Cross and Schlesinger, 1995). However, recent research results have shown that lowland rice drew P from nearly the entire continuum of alkali- and acid-soluble inorganic soil P fractions under P exhaustion conditions (Zhang et al., 2006). In the current experiment, PR application not only significantly increased ryegrass dry weight and P uptake by providing nutrient P during the ryegrass season, but also significantly increased soil available P and NaOH-P fractions after the ryegrass season, which could supply P to rice in next season. Most of the PR-P applied went to HCl-P and residual-P fractions in the soil, which could gradually provide available P to the plants of late seasons (Chien and Menon, 1995). To be practical, the rate of KPR-P application is usually about 5 times that of water-soluble P fertilizers (Meng et al., 2006). The relative agronomic effectiveness (RAE) of KPR250 treatment was about 68% in dry weight and 42% in P uptake with respect to MCP50 by taking RAE of control as 0%. From a practical perspective to guarantee no yield loss in the first several crop seasons, application of PR combined with certain amount of water-soluble P fertilizer might be necessary to maintain a suitable soil available P level. When the soil P status is high, there is no yield response to P application in the first two to three rice crop seasons as in the case of many paddy fields in Zhejiang Province (Zhang and Wang, 1999). Substitution of suitable PR for water-soluble P fertilizers to maintain P status or replenish P uptake and at the same time to reduce eutrophication due to high soil available P was tested for rice production in Zhejiang Province (Wu et al., 2002). It also has been used for pasture production in New Zealand (Bolan and Hedley, 1997). The benefits of this technique need to be further evaluated locally.
The paddy soil used in this study was taken from a long-term field experiment with double rice cropping after seven years of no P treatment, which resulted in low available P. Deficiencies of P limit both plant growth and microbial growth, so its availability is a major determinant of overall soil productivity (Zhang et al., 2005). The results show that ryegrass yield and soil total microbial biomass (PLFAtotal) significantly increased after high rate of KPR (KPR250) and water-soluble P fertilizer (MCP50) applications compared with the low rate of KPR (KPR50) and the control.
PLFAs are widely accepted as biomarkers that indicate viable components of soil microbial biomass and can provide a microbial community “fingerprint” (Liang et al., 2008). PLFA analysis can provide more detailed information of the “active” soil microbial community compared with the culture method (Vestal and White, 1989; Yao et al., 2000; Liang et al., 2008). Application of KPR significantly (P<0.05) increased fungal PLFA as well as the ratio of PLFAfung/PLFAbact in the soil as water-soluble P did. Increased P nutrient availability, enhancement of root growth and exudation may have stimulated fungi (with a wide range of catabolic pathways) to utilize carbon sources unavailable to other microorganisms (Steer and Harris, 2000).
Both the principal component analysis and the cluster analysis of the PLFA data indicated a shift in microbial community under different P treatments compared with the control (no P application). Rooney and Clipson (2009) reported that phosphate addition to grassland soil influenced both fungal and bacterial communities. It was thought that phosphate was an important factor governing overall microbial communities in soil (Rooney and Clipson, 2009; Stutter et al., 2009). In this study, the microbial community changes could be related to changes in the availability of resources, particularly root exudates, and quantitative and qualitative changes in the input of organic substrates that were caused by the improvement of soil P availability (Yeates et al., 1997).
A different soil microbial community was established after water-soluble P fertilizer application compared with KPR and control. Water-soluble P application can immediately produce a higher soil available P level compared with PR because of poor solubility and slow release natures of PR. It was found that the increased P nutrient availability might increase C input to soils through enhanced plant growth and increased fine root turnover (King et al., 2002; Dawson et al., 2003). Relative utilization of this C source is an important driver of microbial group abundance (Paterson et al., 2007). It seems that P availability is an important contributor to the soil total microbial biomass and community diversity.
Acknowledgments
The authors would like to thank Dr. S. H. Chien, Principal Soil Scientist of International Fertilizer Development Center (IFDC), USA, for his kindly revising and editing the manuscript. Thanks also go to Ms. Na Ding of Zhejiang University, China for her assistance in PLFA analysis.
Footnotes
Project supported by Zhejiang Provincial Key Laboratory of Subtropical Soil and Plant Nutrition, and the Key Laboratory of Polluted Environment Remediation and Ecological Health of Ministry of Education, Zhejiang Province, China
References
- 1.Bolan NS, Hedley MJ. Developments in some aspects of reactive phosphate rock research and use in New Zealand. Aust J Exp Agric. 1997;37(8):861–884. doi: 10.1071/EA96104. [DOI] [Google Scholar]
- 2.Bolan NS, White RE, Hedley MJ. A review of the use of phosphate rocks as fertilizers for direct application in Australia and Zealand. Aust J Exp Agric. 1990;30(2):297–313. doi: 10.1071/EA9900297. [DOI] [Google Scholar]
- 3.Bolan NS, Elliott J, Gregg PEH, Weil S. Enhanced dissolution of phosphate rocks in the rhizosphere. Biol Fert Soils. 1997;24(2):169–174. doi: 10.1007/s003740050226. [DOI] [Google Scholar]
- 4.Bossio DA, Scow KM, Gunapala N, Graham KJ. Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microbial Ecol. 1998;36(1):1–12. doi: 10.1007/s002489900087. [DOI] [PubMed] [Google Scholar]
- 5.Bossio DA, Fleck JA, Scow KM, Fujii R. Alteration of soil microbial communities and water quality in restored wetlands. Soil Biol Biochem. 2006;38(6):1223–1233. doi: 10.1016/j.soilbio.2005.09.027. [DOI] [Google Scholar]
- 6.Chien SH. Interpretation of Bary I extractable phosphorus from acid soil treated with phosphate rock. Soil Sci. 1978;144(1):34–39. [Google Scholar]
- 7.Chien SH, Menon RG. Factors affecting the agronomic effectiveness of phosphate rock for direct application. Fert Res. 1995;41(3):227–234. doi: 10.1007/BF00748312. [DOI] [Google Scholar]
- 8.Chien SH, Leon LA, Tejeda H. Dissolution of North Carolina phosphate rock in acid Colombian soils as related to soil properties. Soil Sci Soc Am J. 1980;44:1267–1271. [Google Scholar]
- 9.Cross AF, Schlesinger WH. A literature review and evaluation of the Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma. 1995;64(3-4):197–214. doi: 10.1016/0016-7061(94)00023-4. [DOI] [Google Scholar]
- 10.Dawson LA, Grayston SJ, Murray PJ, Cook R, Gange AC, Ross JM, Pratt SM, Duff EI, Treonis A. Influence of pasture management (nitrogen and lime addition and insecticide treatment) on soil organisms and pasture root system dynamics in the field. Plant Soil. 2003;255(1):121–130. doi: 10.1023/A:1026100330577. [DOI] [Google Scholar]
- 11.Drever JI, Vance GF. Role of Soil Organic Acids in Mineral Weathering Processes. In: Lewan MD, Pittman ED, editors. The Role of Organic Acids in Geological Processes. Berlin: Springer; 1994. pp. 138–161. [Google Scholar]
- 12.Duponnois R, Colombet A, Hien V, Thioulouse J. The mycorrhizal fungus Glomus intraradices and rock phosphate amendment influence plant growth and microbial activity in the rhizosphere of Acacia holosericea . Soil Biol Biochem. 2005;37(8):1460–1468. doi: 10.1016/j.soilbio.2004.09.016. [DOI] [Google Scholar]
- 13.EPA (Environmental Protection Agency) Methods of Chemical Analysis for Water and Wastes. Cincinnati, OH: U.S. Environmental Protection Agency; 1971. [Google Scholar]
- 14.Fairhurst TH, Witt C. Rice: A Practical Guide to Nutrient Management. Singapore and Los Baños: Potash and Phosphate Institute, Potash and Phosphate Institute of Canada, and International Rice Research Institute; 2002. [Google Scholar]
- 15.Hart MR, Quin B, Nguyen ML. Phosphorus runoff from agricultural land and direct fertilizer effects: a review. J Environ Qual. 2004;33:1954–1972. doi: 10.2134/jeq2004.1954. [DOI] [PubMed] [Google Scholar]
- 16.Hedley MJ, Stewart JWB, Chauhan BS. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J. 1982;46:970–976. [Google Scholar]
- 17.Hinsinger P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil. 2001;237(2):173–195. doi: 10.1023/A:1013351617532. [DOI] [Google Scholar]
- 18.Hoffland E, Findenegg GR, Nelemans JA. Solubilization of rock phosphate by rape. II. Local root exudation of organic acids as a response to P starvation. Plant Soil. 1989;113(2):161–165. doi: 10.1007/BF02280176. [DOI] [Google Scholar]
- 19.King JS, Albaugh TJ, Allen HL, Buford M, Strain BR, Dougherty P. Below-ground carbon input to soil is control led by nutrient availability and fine root dynamics in loblolly pine. New Phytol. 2002;154(2):389–398. doi: 10.1046/j.1469-8137.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 20.Liang C, Fujinuma R, Balser TC. Comparing PLFA and amino sugars for microbial analysis in an Upper Michigan old growth forest. Soil Biol Biochem. 2008;40(8):2063–2065. doi: 10.1016/j.soilbio.2008.01.022. [DOI] [Google Scholar]
- 21.Lovell RD, Jarvis SC, Bardgett RD. Soil microbial biomass and activity in long-term grassland: effects of management changes. Soil Biol Biochem. 1995;27(7):969–975. doi: 10.1016/0038-0717(94)00241-R. [DOI] [Google Scholar]
- 22.Mehra OP, Jackson ML. Iron oxide removed from soils and clays by a dithionite-citrate system buffer with sodium bicarbonate. Clay Clay Miner. 1960;7(3):317–329. [Google Scholar]
- 23.Meng CF, Cao ZH, Jiang PK, Xu QF. Application of phosphate rock in rapeseed-rice cropping system on an acid paddy soil in central Zhejiang. Acta Pedol Sin. 2006;43(4):599–604. (in Chinese) [Google Scholar]
- 24.Murphy J, Riley JR. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27(1):31–36. doi: 10.1016/S0003-2670(00)88444-5. [DOI] [Google Scholar]
- 25.Murray PJ, Cook R, Currie AF, Dawson LA, Gange AC, Grayston SJ, Treonis AM. Interactions between fertilizer addition, plants and the soil environment: Implications for soil faunal structure and diversity. Appl Soil Ecol. 2006;33(2):199–207. doi: 10.1016/j.apsoil.2005.11.004. [DOI] [Google Scholar]
- 26.Myers RT, Zak DR, White DC, Peacock A. Landscape-level patterns of microbial community composition and substrate use in upland forest ecosystems. Soil Sci Soc Am J. 2001;65:359–367. [Google Scholar]
- 27.Olsen SR, Cole CV, Watanabe FS, Dean LA. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Dept Agric Circ. 1954;939:19. [Google Scholar]
- 28.Paterson E, Gebbing T, Abel C, Sim A, Telfer G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol. 2007;173(3):600–610. doi: 10.1111/j.1469-8137.2006.01931.x. [DOI] [PubMed] [Google Scholar]
- 29.Rooney DC, Clipson NJW. Phosphate addition and plant species alters microbial community structure in acidic upland Grassland soil. Microb Ecol. 2009;57(1):4–13. doi: 10.1007/s00248-008-9399-2. [DOI] [PubMed] [Google Scholar]
- 30.Shigaki F, Sharpley A, Prochnow LI. Source-related transport of phosphorus in surface runoff. J Environ Qual. 2006;35(6):2229–2235. doi: 10.2134/jeq2006.0112. [DOI] [PubMed] [Google Scholar]
- 31.Steer J, Harris JA. Shifts in the microbial community in rhizosphere and non-rhizosphere soils during the growth of Agrostis stolonifera . Soil Biol Biochem. 2000;32(6):869–878. doi: 10.1016/S0038-0717(99)00219-9. [DOI] [Google Scholar]
- 32.Stutter MI, Langan SJ, Lumsdon DG. Vegetated buffer strips can lead to increased release of phosphorus to waters: a biogeochemical assessment of the mechanisms. Environ Sci Technol. 2009;43(6):1858–1863. doi: 10.1021/es8030193. [DOI] [PubMed] [Google Scholar]
- 33.Tambunan D, Hedley MJ, Bolan NS, Turner M. A comparison of sequential extraction procedures for measuring phosphate rock residues in soils. Fert Res. 1993;35(3):183–191. doi: 10.1007/BF00750637. [DOI] [Google Scholar]
- 34.Toyota K, Kuninaga S. Comparison of soil microbial community between soils amended with or without farmyard manure. Appl Soil Ecol. 2006;33(1):39–48. doi: 10.1016/j.apsoil.2005.09.002. [DOI] [Google Scholar]
- 35.Turpeinen R, Kairesalo T, Häggblom MM. Microbial community structure and activity in arsenic-, chromium-, and copper-contaminated soils. FEMS Microbiol Ecol. 2004;47(1):39–50. doi: 10.1016/S0168-6496(03)00232-0. [DOI] [PubMed] [Google Scholar]
- 36.Vestal JR, White DC. Lipid analysis in microbial ecology: quantitative approaches to the study of microbial community. BioScience. 1989;39(8):535–541. doi: 10.2307/1310976. [DOI] [PubMed] [Google Scholar]
- 37.Wu CS, Meng CF, Lu XN, Teng CQ, Zhao SR. The agronomic availability and economic effect of applying phosphate rock to acid paddy soil. Phosphate Comp Fertil. 2002;17(3):67–69. (in Chinese) [Google Scholar]
- 38.Yao HY, He ZL, Wilson MJ, Campbell CD. Microbial biomass and community structure in a sequence of soil with increasing fertility and changing land use. Microb Ecol. 2000;40(3):223–237. doi: 10.1007/s002480000053. [DOI] [PubMed] [Google Scholar]
- 39.Yeates GW, Bardgett RD, Cook R, Hobbs PJ, Bowling PJ, Potter JF. Faunal and microbial diversity in three Welsh grassland soils under conventional and organic management regimes. J Appl Ecol. 1997;34(2):453–471. doi: 10.2307/2404890. [DOI] [Google Scholar]
- 40.Zapata F, Zaharah AR. Phosphorus availability from phosphate rock and sewage sludge as influence by the addition of water soluble phosphate fertilizer. Nutr Cycl Agroecosys. 2002;63(1):43–48. doi: 10.1023/A:1020518830129. [DOI] [Google Scholar]
- 41.Zhang QC, Wang GH, Fang B. Influence of fertilizations treatments on nutrient uptake by rice and soil ecological characteristics of soil microorganism in paddy field. Acta Pedol Sin. 2005;42(1):116–121. (in Chinese) [Google Scholar]
- 42.Zhang QC, Wang GH, Feng YK, Sun QZ, Witt C, Dobermann A. Changes in soil phosphorus fractions in a calcareous paddy soil under intensive rice cropping. Plant Soil. 2006;288(1-2):141–154. doi: 10.1007/s11104-006-9100-9. [DOI] [Google Scholar]
- 43.Zhang ZJ, Wang GH. Paddy soil phosphorus status and its environmental effects evaluation in Jiaxing area. Bull Sci Technol. 1999;15(5):377–381. (in Chinese) [Google Scholar]