Abstract
Purpose
No therapy has ever demonstrated prolongation of survival in stage IV metastatic melanoma. The association of cytokine-induced autoimmunity with improved prognosis led us to investigate the impact of multi-epitope melanoma vaccines alone and in combination with cytokines in this Eastern Cooperative Oncology Group multicenter phase II trial.
Experimental Design
Eligible patients were required to have failed prior therapies and are HLA-A2 positive. Three HLA class I restricted lineage antigen epitopes were administered in a factorial 2 × 2 design. Peptide vaccine alone (arm A), or combined with GM-CSF (Immunex, Seattle, WA) 250 µg subcutaneously daily for 14 out of 28 days each month (arm B), or combined with IFNα2b (Intron A, Schering-Plough, Kenilworth, NJ) 10 million units per m2 three times a week (arm C), or in combination with both IFNα2b and GM-CSF (arm D). The primary endpoint was immune response measured by ELISPOT immunoassay; secondary endpoints were clinical antitumor response, disease-free and overall survival.
Results
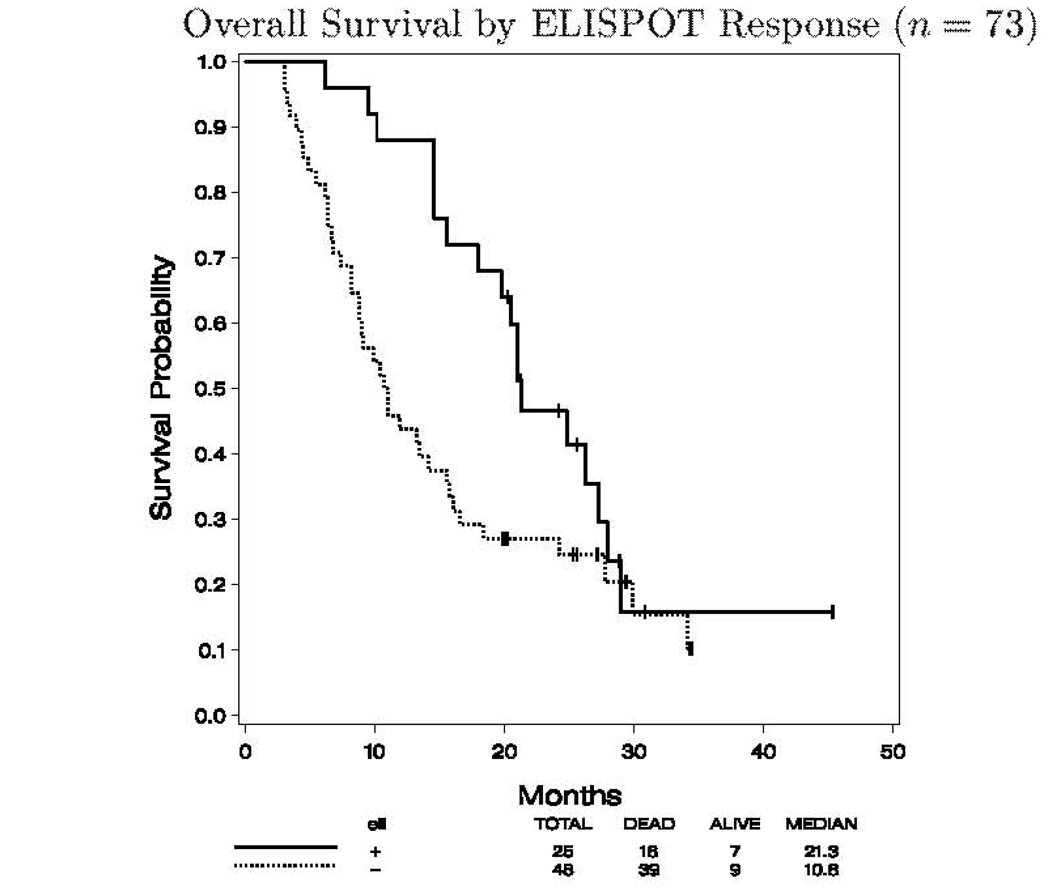
120 patients enrolled and 115 patients were analyzed. Immune responses to at least one melanoma antigen were observed in 26/75 (35%) patients. Neither IFNα2b nor GM-CSF significantly improved immune responses. Six objective clinical responses were documented. At a median follow-up of 25.4 months the median overall survival of patients with vaccine immune response was significantly longer than that of patients with no immune response (21.3 versus 13.4 months; p=.046).
Conclusion
Immune response to vaccination correlates with prolonged survival in patients with metastatic melanoma and is not enhanced by immunomodulatory cytokines tested in this trial.
INTRODUCTION
No therapy has ever shown a significant impact upon overall survival in patients with metastatic melanoma. Only high-dose bolus interleukin-2 (IL-2) has been approved by the U.S. Food and Drug Administration for therapy of advanced melanoma on the basis of durable responses induced in 4.7–6% of patients (1). Furthermore, in the adjuvant setting of operable high-risk melanoma rigorous randomized phase III trials of high-dose IFNα2b (HDI) have demonstrated both consistent relapse-free (RFS) and overall survival (OS) benefit in two of these trials (2). Granulocyte-monocyte colony stimulating factor (GM-CSF) is another cytokine which has suggested DFS and OS benefit in a single phase II study of patients with operable resected stage III and stage IV melanoma compared with historic controls (3). These observations led us to design an intergroup trial of GM-CSF given in monthly cycles for treatment of patients with resectable advanced stage III and limited stage IV melanoma (4).
The common denominator of therapies of benefit in melanoma has been the augmentation of host immunity. High-dose IFN given in the neoadjuvant setting induces higher increases in the number of tumor-infiltrating CD3+ and CD11c+ immune cells in clinical responders compared to non-responders (5). Similarly, high-dose bolus IL-2 induces inflammation at the tumor site (6) whereas GM-CSF has potent effects upon myeloid dendritic cells (7) (8). These cytokines have been shown to induce autoimmunity that is associated with clinical benefit (9) (10) (11). Concurrent or sequential administration of cytokines with melanoma vaccines in small studies have suggested augmented immunologic response in association with clinically significant antitumor responses (12) (13).
Vaccines have become the focus of major investigative efforts across the world, driven by the expanding understanding of the immunogenicity of melanoma antigens (14). Most previously reported vaccine studies have been conducted at single institutions, employing one or a limited panel of peptide antigens, with a variety of immunological adjuvants, resulting in occasional clinical benefit (15) (16) (17) (18) (19). These limited benefits have been attributed to regulatory T cells, the low frequencies of antigen-specific T cells, and to defects in immune cell trafficking and function (20). A critical need has therefore developed for well-powered randomized studies testing the immunodominant melanoma peptide epitopes along with novel vaccination strategies to produce higher frequencies and more durable immune responses. No cooperative group trial has yet evaluated the immunogenicity of vaccines comprised of multiple lineage antigens, or compared vaccination alone, vs. in combination with immunomodulatory cytokines.
E1696 was designed to test immune responses of peptide-specific CD8+ T-cell precursors by ELISPOT assay against epitopes derived from three lineage-restricted antigens MART-1, gp100, and tyrosinase, in patients with metastatic unresectable melanoma. The peptide epitopes were selected based on the high frequency of the anti-melanoma cytotoxic T-cell clones derived from HLA-A2+ tumor-infiltrating lymphocytes against antigenic sequences identified by both cDNA expression cloning and protein biochemical approaches (21) (22) (23) (24) (25) (26) (27) (28) (18). Given the promise of two immunomodulatory biological agents available in the outpatient setting, this study was also designed to evaluate the immunomodulatory effects of GM-CSF, IFN or the combination of these two agents, upon vaccine immune response and toxicity. Finally, analyses of anti-tumor responses and correlations of immune response with survival outcome was explored in this multicenter randomized trial.
PATIENTS AND METHODS
Patients
Eligible patients had histologically confirmed Stage IV melanoma (AJCC 5th edition), absent brain metastases by magnetic resonance imaging (MRI) or computerized tomography (CT) scans within 4 weeks prior to randomization, and measurable disease. Prior treatments were allowed if administered more than four weeks prior to randomization, including IFN or GM-CSF. Patients needed to have an ECOG performance status of 0 or 1, adequate hematologic (WBC ≥ 4.000 /mm3, platelet count ≥ 100,000 /mm3 and lymphocytes > 700 /mm3), hepatic (AST, serum bilirubin, alkaline phosphatase, and LDH ≤ 2 times institutional upper limit) and renal (serum creatinine ≤ 1.8 mg/dL) function within 4 weeks prior to randomization and be HLA-A2 positive, by serologic or genotypic analysis. Patients with HIV, other current malignancies, active autoimmune disorders, or other conditions requiring chronic corticosteroid use were excluded. All patients provided written informed consent, and the study was approved by the Institutional Review Board of each participating ECOG-affiliated institution.
Peptides
HLA-A2 restricted peptides used in this study included: AAGIGILTV (MART-127–35) (27), the enhanced gp100 peptide analog (gp100 209, T210M) IMDQVPFSV (gp100209–217) (18),and the tyrosinase peptide analog [tyrosinase 368–376 (370D) [NSC # 699048]] YMNGTMSQV (tyrosinase368–376) (22). The peptides were synthesized, placed in vials containing 1 mL of a sterile 1mg/mL solution for injections. All vaccine peptides and those used for ELISPOT assays were provided by the NCI/Cancer Therapy Evaluation Program (CTEP) as Investigational New Drug #6123.
Immunization Protocol
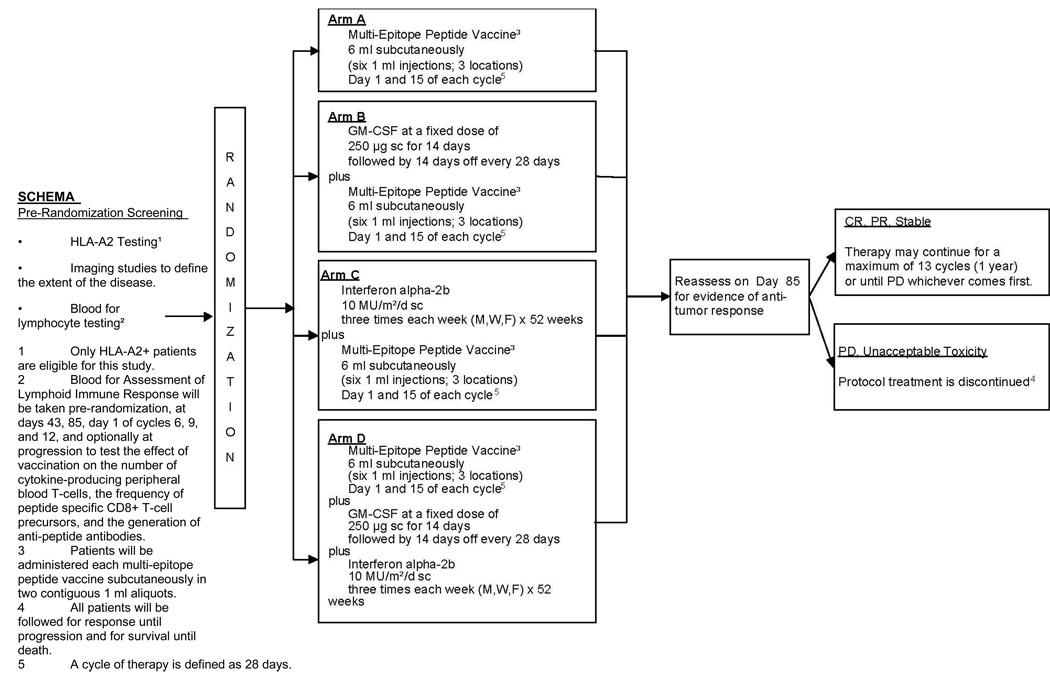
The schema of this study is illustrated in Figure 1. After patients provided written informed consent, immunophenotypic or genotypic analysis for HLA-A2 status and radiographic studies were performed. If HLA-A2 positive, additional peripheral blood was obtained for baseline immunologic assays, and patients were randomized at the ECOG operations office, by telephone or through ECOG web registration with equal weighting into one of the four treatment arms:
Arm A, Multi-Epitope Peptide Vaccine Alone Patients were administered each multi-epitope peptide vaccine (6 injections, 3 locations) subcutaneously (SC) using rotating truncal sites close to nodal drainage groups of the 4 extremities on days 1 and 15 of each cycle (28 days) for up to 13 cycles (1 year). Each peptide was emulsified with Montanide ISA-51 (Seppic, France) and administered in two 1 mL SC aliquots. Vaccine was injected the same way in all treatment arms.
Arm B, GM-CSF + Multi-Epitope Peptide Vaccine Patients self-administered GM-CSF (Immunex, Seattle, WA) at a fixed dose of 250 µg SC daily for 14 days followed by 14 days off every 28 days for one year (13 cycles) or until disease progression. We used the previously established dose (3). To ensure compliance patients were requested to complete the E1696 Patient Diary. GM-CSF dose was permanently reduced by 50% at the beginning of the next cycle for WBC ≥ 60,000/mm3.
Arm C, Interferon α-2b + Multi-Epitope Peptide Vaccine IFN (IFNα-2b, Schering-Plough, Kenilworth, NJ) was administered at 10 MU/m2 SC three times a week (Monday, Wednesday, Friday) for 52 weeks, or until disease progression. Similar to Arm B, patients were obliged to complete the Patient Diary. The dose chosen was that established for maintenance phase high-dose interferon adjuvant therapy. Criteria for IFN dose reduction were adopted from prior adjuvant studies (29).
Arm D, IFNα-2b + GM-CSF + Multi-Epitope Peptide Vaccine Both IFNα-2b and GM-CSF were administered in the exact same schedule as in Arms B and C, respectively.
The duration of therapy for all arms was 13 cycles (52 weeks). Patients who did not progress during treatment were followed every 3 months to 24 months and every 6 months to 60 months following study enrollment with physical exam and whole body CT scans.
Fig. 1.
Schema for ECOG phase II trial E1696.
HLA Typing
HLA typing was performed either genotypically using polymerase chain reaction (HLA-0201) or serologically utilizing multiple antibodies (MA2.1, W6.32, BB7.2, L243) for HLA-A2 with the respective isotype controls in the Immunological Monitoring Laboratory of ECOG at the University of Pittsburgh Cancer Institute. For genotypic analysis we used polymerase chain reaction methods (HLA-A2 0201).
Enzyme-linked Immunospot (ELISPOT) Assays
Peripheral blood from patients was sent by overnight courier to the ECOG Core Immunological Reference Laboratory at the University of Pittsburgh Cancer Institute. Peripheral blood mononuclear cells were isolated by a Ficoll gradient centrifugation and were cryopreserved. After completion of the vaccine regimen, samples from baseline and the day 43 and 85 time points were evaluated simultaneously by an interferon-gamma (IFNγ) (ELISPOT) assay (30). No in vitro sensitization of lymphocytes to enhance detection of low-frequency precursor T-cells directed against melanoma lineage and other antigens was performed, as this has been utilized for various intervals in multiple prior studies. We chose instead to avoid the artifacts associated with in vitro priming in this study where evaluation of T-cell responses was based on previously published definitions (16). The IFNg ELISPOT was performed by carefully trained technologists, utilizing standardized SOPs and validated reagents. Performance of different technologists involved in assay conduct were cross-compared. All plates were read by automated ELISPOT plate readers. Each assay contained wells in which normal healthy donor cells (made in large, multi-use batches) were tested for spontaneous and PMA/Ionomycin-stimulated cytokine release, serving as an assay control. Medium-only wells served as controls for non-specific spots from other reagents.
Patient cells stimulated with OKT3 served as patient cell function controls, over and above %recovery and %viability measures. Recall responses to Flu peptide from CD8+ cells, and non-specific responses to Ova were also included. Wells were plated in triplicate and standard deviations were measured. All samples were assayed on the same plate on the same day to avoid inter-assay variation. A number of internal controls were utilized to test for spontaneous release of IFNg as well as stimulation to test for the ability to mount an immune response. For example, test wells were plated with the responders alone, responders + APC (no peptide), and responders + Ova protein to test for spontaneous IFNg production in the absence of a stimulus. In addition, test wells were plated with responders + OKT3, and a set of wells with Flu peptide to insure an immune response could be elicited. All reagent lot numbers were tested prior to patient assays for quality assurance.
Evaluation of Clinical Response and Statistical Considerations
Objective antitumor response was evaluated in measurable disease by CT or MRI scans and by clinical measurement of cutaneous lesions using the standard ECOG tumor response criteria (ECOG Policies and Procedures Manual).
The primary endpoint of the study was peptide-specific immune response by treatment (IFN vs. no IFN, GM-CSF vs. no GM-CSF) in a 2 × 2 factorial design with IFN on one axis and GM-CSF on the other axis. ELISPOT assays were the sole measure of treatment-induced immune modulation in this study. For an increase of immunological response from 5% in non-IFN or non-GM-CSF treatment arms to 30% in IFN or GM-CSF treatment arms, 18 fully evaluable patients per treatment arm and a two-sided significance level of 10%, the power to detect such a difference using Fisher's exact test was 81%. The secondary endpoints of the study were assessment of PFS and OS for patients in all four treatment arms.
In analyzing the time-to-event data, the method of Kaplan-Meier (K–M) was used and significant differences observed were tested by the log-rank test. The Cox proportional hazard model was used for a multivariate time-to-event analysis. Fisher's exact test was used to compare proportions. All p-values were reported for two-sided tests.
RESULTS
Accrual
This study accrued 120 patients in 7 major institutions of ECOG between September 22, 2000 and May 14, 2003. One patient randomized to arm B never received any therapy due to insurance issues. 4 patients were ineligible. For E1696 115 cases were included in the analysis of clinical outcomes, whereas toxicity data were summarized for all 117 treated cases. 75 patients had ELISPOT data taken at baseline and at least one later time point (day 43 or day 85). The stage breakdown for 72 of 75 patients with full staging data was M1a, 15 (20.8%), M1b, 30 (41.7%) and M1c, 27 (37.5).
Patient Characteristics and Immunologic Response
Table 1a displays the on-study characteristics of the 115 treated patients who were analyzable for immune response. 75 patients had ELISPOT data at baseline and for at least one later time point. Of these 75 patients, 66 patients had ELISPOT data at day 43 and 54 patients at day 85. Table 1b summarizes immune responses by ELISPOT data for each lineage-restricted melanoma antigen. The immune response rate to at least one of the peptides (overall immune response rate, OIRR) was 21.2% at day 43 and 37% at day 85. The OIRR at day 43 or day 85 was 34.7% (95 CI: 24%, 46.6%). The immune response rate to gp100 was the highest as compared with MART-1 and tyrosinase. There was a marginally significant association between the OIRR and the disease stage (AJCC, 6th edition) upon study enrollment (46.7% and 43.3% in patients with M1a and M1b disease, respectively, versus 20.7% in patients with M1c disease, p=0.112). Neither IFN nor GM-CSF significantly increased the immune response rate. OIRR was 16/37 (43.2%) with IFN treatment in comparison to 9/36 (25%) without IFN treatment. By GM-CSF treatment, OIRR was 13/34 (38.2%) with GM-CSF and 12/39 (30.8%) without GM-CSF.
Table 1.
On-Study Characteristics (n=115)
| n | (%) | |
|---|---|---|
| Gender | ||
| Male | 69 | (60%) |
| Female | 46 | (40%) |
| Race (n=110) | ||
| White | 110 | (100%) |
| Performance Status (n=113) | ||
| 0 | 74 | (65.5%) |
| 1 | 39 | (34.5%) |
| Depth of Invasion (n=80) | ||
| I | 1 | (1.3%) |
| II | 8 | (10.0%) |
| III | 22 | (27.5%) |
| IV | 32 | (40.0%) |
| V | 17 | (21.3%) |
| Primary Tumor Site (n=106) | ||
| Head and Neck | 19 | (17.9%) |
| Upper Limb | 5 | (4.7%) |
| Lower Limb | 17 | (16%) |
| Trunk | 46 | (43.4%) |
| Anagenital | 2 | (1.9%) |
| Ocular | 8 | (7.6%) |
| Mucosal | 4 | (3.8%) |
| Other | 5 | (4.7%) |
| Histologic Type (n=79) | ||
| Amelanotic | 6 | (7.6%) |
| Melanotic | 73 | (92.4%) |
| Sites of Distant Metastasis | ||
| (Visceral Involvement) | Measurable (n) | Non-measurable (n) |
| Lung | 70 | 11 |
| Pleura | 1 | 1 |
| Liver | 24 | 5 |
| Brain/CNS | 0 | 1 |
| Bone | 0 | 6 |
| Other Visceral | 18 | 6 |
| Sites of Distant Metastasis | ||
| (Non-visceral Involvement) | Measurable (n) | Non-measurable (n) |
| Distant nodes | 29 | 5 |
| Soft tissue | 23 | 6 |
| Skin | 19 | 2 |
| Other non-visceral | 12 | 4 |
| AJCC M Stage (n=111) | ||
| M1a | 20 | (18%) |
| M1b | 47 | (42.4%) |
| M1c | 44 | (39.6%) |
| Age (years) | ||
| Median | 62 | |
| Age (years) | 23 – 83 | |
| Breslow Thickness (mm) (n=81) | ||
| Median | 2.5 | |
| Range | 28 – 24 | |
| Prior Treatment | ||
| Surgery | 109 | |
| Chemotherapy | 24 | |
| Radiotherapy | 22 | |
| Immunotherapy | 44 | |
| Hormonal Therapy | 1 | |
| Gene Therapy | 1 | |
| Other | 9 |
Accrual by Institution > 5%
1). University of Pittsburgh, 23.3%; 2). University of Wisconsin, 10.00%; 3). Mayo Clinic Rochester, 10.0%;
4). University of Pennsylvania, 8.3%; 5). Johns Hopkins University, 7.5%; 6). Beth Israel Deaconess Medical Center, 6.7%;
7). Emory University, 6.7%.
Clinical Antitumor Responses
Of the 115 analyzable patients, 2 patients had a complete response (CR), 4 had a partial response (PR), 30 had stable disease (SD), and 73 had progressive disease (PD) while 6 patients were non-evaluable. Table 2a and 2b summarize the baseline characteristics of the 6 patients with CR or PR, and the correlation of clinical and immunological response. There was no significant difference across treatment arms in terms of best overall response, defined as non-progression (CR plus PR plus SD). As expected, only AJCC staging of disease upon study enrollment was significantly correlated with best overall response (13/20 (65%) for M1a patients versus 12/47 (25.5%) for M1b patients versus 11/44 (25%) for M1c patients; p=.04).
Table 2.
Immune Response by Peptide (n = 75)
| Day | GP 100 | MART-1 | Tyrosine |
|---|---|---|---|
| Day 43 | 12.3% (8/65) | 9.1% (6/66) | 5.1% (3/59) |
| Day 85 | 28% (14/50) | 5.6% (3/54) | 10.6% (5/47) |
| Overall Response to Any of Three Peptides | |||
| Day 43 | 21.2% (14/66) | ||
| Day 85 | 37% (20/54) | ||
| Day 43 or Day 85 | 34.7% (26/75) | ||
Survival
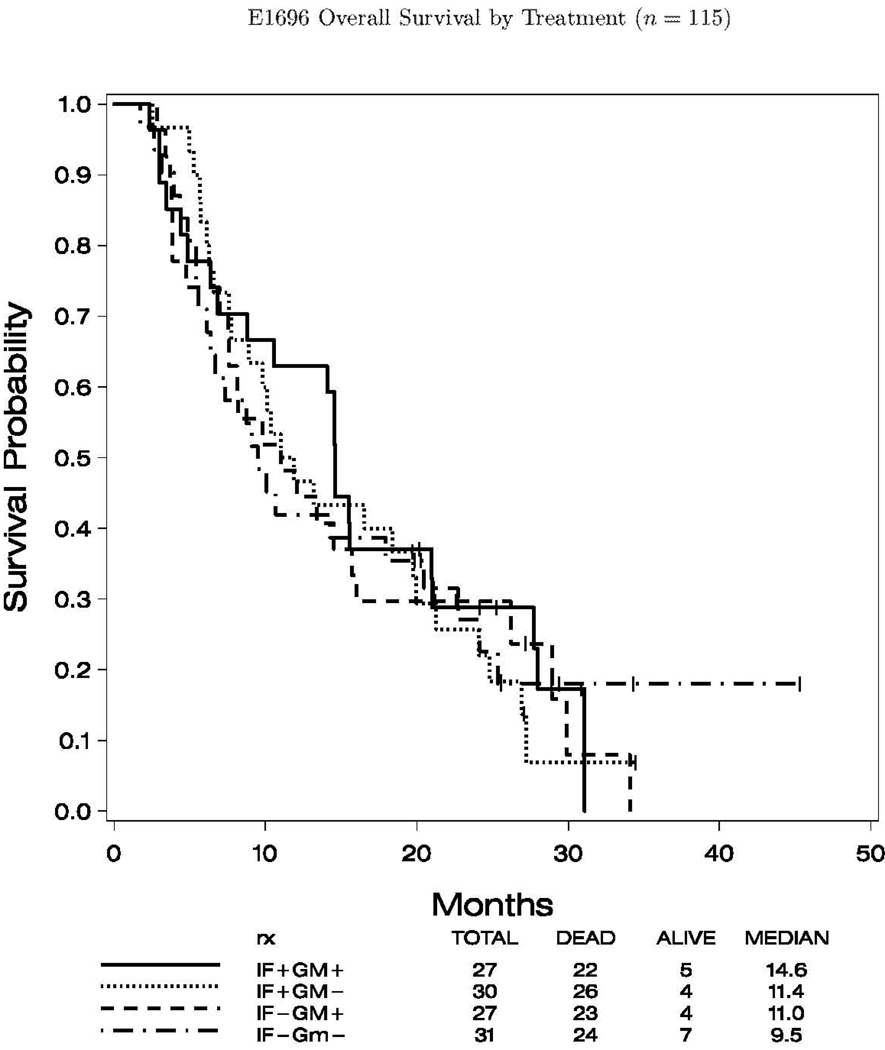
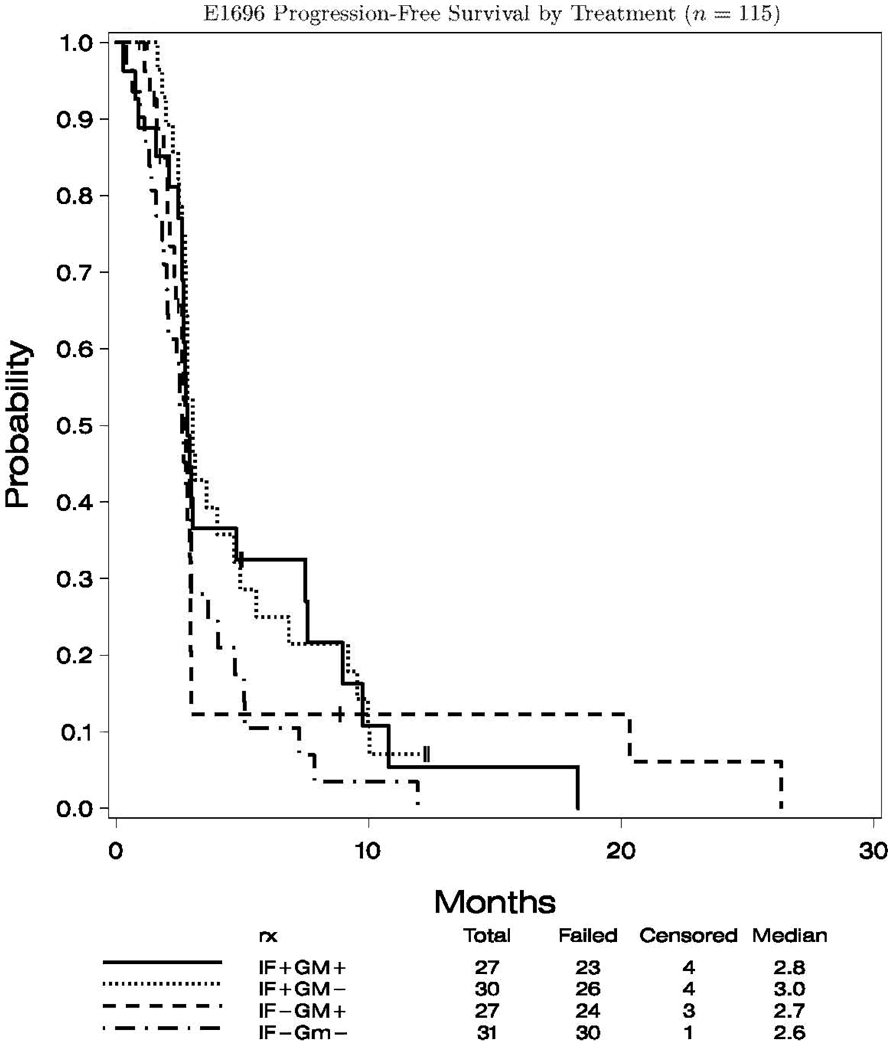
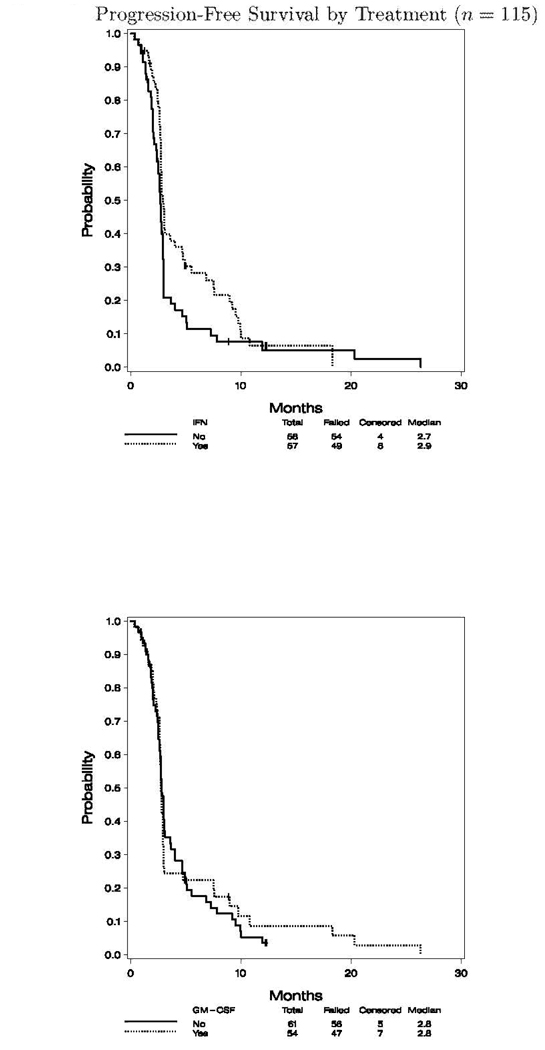
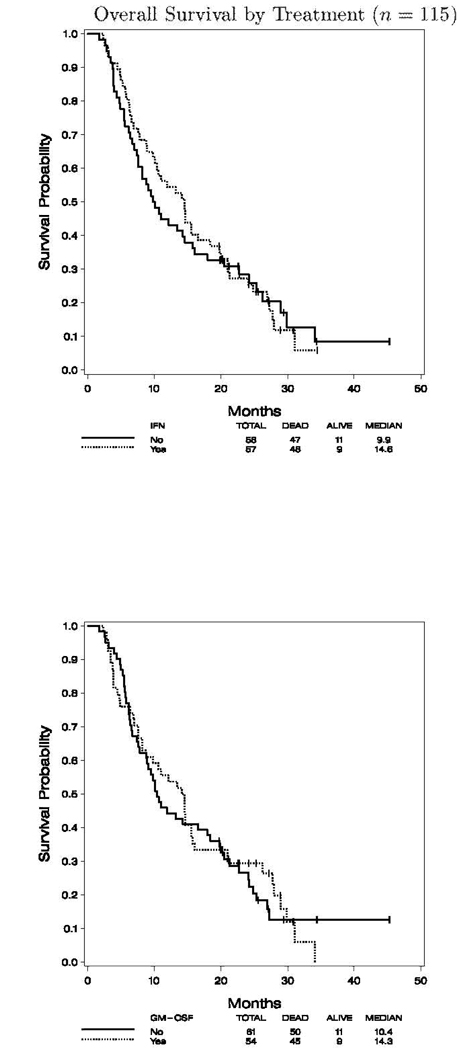
The median follow up for the 115 analyzable cases was 25.4 months (range 19.7, 45.3) to final analysis. For the entire study cohort the median progression-free survival (PFS) was 2.8 months (95% CI: 2.7, 3.0), and the overall survival (OS) was 11.9 months (95% CI: 9.5, 14.6). Figures 2.1 and 2.2 show the K–M plots for PFS, and OS by treatment status, respectively. There was no significant difference in OS by IFN- and/or GM-CSF treatment status (data not shown).
Fig. 2.
PFS and OS by treatment (n = 115) and PFS by treatment (n = 115). 1, A, progression of survival of patients according to treatment with IFN-α2b; B, progression of survival of patients according to treatment with GM-CSF. OS by treatment (n = 115). 2, A, OS by treatment with IFN-α2b; B, OS by treatment with GM-CSF.
Associations between Immune and Clinical Data
The immune response data was associated with clinical data. Of the 75 patients with available ELISPOT data, 73 patients were clinically analyzable. Out of these 73 patients, 25 patients had an immune response at any of the two time-points studied (day 43 or day 85), and to at least one of the three peptides administered.
There was a significant difference in OS by immune response status. Immune responders lived longer than the non-immune responders (median OS 21.3 versus 10.8 months, p=0.033). The Cox model was used to evaluate the significance of immune-response status for OS while adjusting for age, sex, PS, AJCC stage at diagnosis, primary disease information (including Breslow depth, nodal involvement, metastatic involvement) and ulceration status. Due to missing data on primary disease information, the total sample size for this analysis was smaller (n=44). As expected, AJCC stage at diagnosis was the most significant predictor of OS (p=0.002). Immune-response status was marginally significant (p=0.073). No other covariates were significant. The K–M plot for OS by immune response status is displayed in Figure 3. No significant difference in PFS by immune response status was noted (data not shown). The data regarding immune response in relation to clinical response have been summarized in Table 2b.
Fig. 3.
OS by ELISPOT response (n = 73). OS according to immune response to one or more of the three peptide vaccine antigens measured by ELISPOT.
Toxicity
Table 3 summarizes treatment-related toxicities based on 117 evaluable patients. No treatment-related deaths occurred. Grade 3 or higher treatment-related toxicity rates were 41.9%, 48.3%, 83.3% and 48.7% for arms A, B, C and D, respectively. As expected, Arm C had a significantly higher rate of toxicity with lymphopenia (9/30), neutropenia (7/30), and fatigue (11/30) being the most frequent.
Table 3.
Treatment-Related Toxicity (n=117)
| Toxicity Type | Arm A (n=31) | Arm B (n=29) | Arm C (n=30) | Arm D (n=27) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | Grade | Grade | |||||||||
| 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | |
| Hemoglobin | 2 | - | - | - | - | - | - | - | - | - | - | - |
| Leukocytes | 1 | - | - | - | - | - | 3 | - | - | - | - | - |
| Lymphopenia | 2 | - | - | 5 | - | - | 9 | - | - | 7 | - | - |
| Neutrophils | - | 1 | - | 1 | - | - | 7 | - | - | 1 | - | - |
| Transfusion: pRBCs | - | - | - | 1 | - | - | - | - | - | 1 | - | - |
| Supraventricular arrhythmias | 1 | - | - | 1 | - | - | 1 | - | - | - | - | - |
| Vasovagal episode | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Cardiac-ischemia | - | - | - | - | - | - | - | - | - | - | 1 | - |
| Edema | 1 | - | - | - | 1 | - | - | - | - | 1 | - | - |
| Hypertension | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Hypotension | - | - | - | - | - | - | - | - | - | 2 | - | - |
| Pericardial effusion/pericarditis | - | - | - | - | - | - | - | 1 | - | - | - | - |
| Thrombosis/embolism | 1 | - | - | - | - | - | - | - | - | 1 | - | - |
| Fatigue | 2 | - | - | - | - | - | 10 | 1 | - | 4 | - | - |
| Rigors/chills | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Weight loss | - | - | - | - | - | - | - | - | - | 1 | - | - |
| DIC | - | - | - | - | - | - | - | - | - | - | 1 | - |
| PTT | 1 | - | - | - | - | - | - | - | - | - | - | - |
| PT | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Injection site reaction | - | - | - | - | - | - | 1 | - | - | 2 | - | - |
| Rash/desquamation | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Wound - infectious | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Anorexia | - | - | - | - | - | - | 2 | - | - | - | - | - |
| Colitis | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Constipation | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Dehydration | 1 | - | - | - | - | - | - | - | - | 1 | - | - |
| Ileus | - | - | - | 1 | - | - | - | - | - | - | 1 | - |
| Nausea | 1 | - | - | - | - | - | - | - | - | 1 | - | - |
| Stomatitis | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Vomiting | 1 | - | - | - | - | - | 1 | - | - | 2 | - | - |
| Diarrhea w/o prior colostomy | - | - | - | - | - | - | 2 | - | - | 1 | - | - |
| CNS hemorrhage | - | 1 | - | - | - | - | - | - | - | - | - | - |
| SGPT | - | - | - | - | - | - | 1 | - | - | - | - | - |
| Infection w/unknown ANC | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Infection w/o neutropenia | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Infection-other | - | - | - | - | - | - | 1 | - | - | - | - | - |
| Lymphatics | 2 | - | - | - | - | - | - | - | - | - | - | - |
| Hyperglycemia | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Hypokalemia | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Hyponatremia | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Hypophosphatemia | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Joint, muscle, bone- other | - | - | - | - | - | - | 1 | - | - | - | - | - |
| Confusion | 1 | 1 | - | - | - | - | - | - | - | - | - | - |
| Dizziness/lightheadedness | - | - | - | - | - | - | 1 | - | - | 3 | - | - |
| Hallucinations | 1 | - | - | - | - | - | 1 | - | - | - | - | - |
| Depression | - | 1 | - | - | - | - | 2 | - | - | - | - | - |
| Neuropathy-motor | 1 | 1 | - | - | - | - | 1 | - | - | - | - | - |
| Neuropathy-sensory | - | - | - | - | - | - | 1 | - | - | - | - | - |
| Syncope | - | - | - | - | - | - | 3 | - | - | 1 | - | - |
| Vertigo | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Ocular-other | - | - | - | - | - | - | - | 1 | - | - | - | - |
| Abdominal pain | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Arthralgia | - | - | - | 1 | - | - | - | - | - | 1 | - | - |
| Bone pain | 1 | 1 | - | 3 | - | - | - | - | - | - | - | - |
| Headache | - | - | - | - | - | - | 2 | - | - | - | - | - |
| Myalgia | - | - | - | 1 | - | - | - | - | - | 1 | - | - |
| Neuropathic pain | 1 | - | - | - | 1 | - | - | - | - | - | - | - |
| Pleuritic pain | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Pain-other | 2 | - | - | - | - | - | - | - | - | 1 | - | - |
| Dyspnea | - | 1 | - | 1 | 2 | - | 2 | - | - | - | 1 | - |
| Hypoxia | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Pneumonitis/pulmonary infiltrates | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Pulmonary-other | 1 | - | - | - | - | - | - | - | - | - | - | - |
| WORST DEGREE | 8 | 5 | - | 11 | 3 | - | 22 | 3 | - | 13 | 3 | - |
DISCUSSION
This study is the first large, randomized, multi-institutional trial of multi-epitope peptide vaccination for patients who have failed prior therapy for metastatic unresectable melanoma. The study addresses laboratory as well as traditional clinical endpoints that were considered critical to future vaccine development. Previous large randomized multicenter melanoma vaccine trials either could not evaluate therapeutic impact because they were conducted in the adjuvant setting (31) (32) (33) or they have not generally employed current immunological monitoring against defined immunogenic peptides (34). We tested the immune response to lineage antigen-derived peptides administered either alone or in combination with two immunomodulatory agents whose efficacy has been or is being tested in the adjuvant clinical arena. We have demonstrated the feasibility and safety of multicenter vaccine studies in conjunction with laboratory immunologic analyses for patients with metastatic melanoma.
The primary endpoints of this study were to define the immune response rate to the multi-epitope peptide vaccination, and to determine the influence of immunomodulatory agents upon peptide-specific immune response. The OIRR was lower compared with prior multi-epitope peptide vaccination studies in patients with unresectable metastatic melanoma (15) (16). This inferior immune response rate may be a laboratory artifact and could be attributable to the in vitro sensitization of lymphocytes to enhance detection of low-frequency melanoma antigen specific precursor T-cells.
This study was large enough to have adequate statistical power to confirm observations from smaller vaccine studies previously reported in metastatic melanoma. First, the objective response rate (CR plus PR) observed in this study was similar to reported results (20) suggesting limited epitope spreading, with insufficient duration and/or magnitude of immune responses induced to achieve tumor regression. Second, gp100209–217 induced higher rates of vaccination response than the other two lineage-specific peptides, and this high immunogenicity has previously been reported in other studies (35) (36). There are two possible explanations for this observation: a higher immunogenicity of the gp100209–217 peptide analog has been noted and an increased abundance of gp100+ melanoma cells lines has been described (37). However, no enhancement of immunogenicity was observed with the modified tyrosinase peptide analog used in this study. Third, the survival of HLA-A2 positive patients enrolled into this trial was longer than historically anticipated (38) (39). HLA-A2 positive patients have been noted to have improved outcome in the setting of operable high-risk disease (40). The prolonged OS observed in this series of patients, all of whom were HLA-A2 positive, will be of interest to compare with patients other series of patients with metastatic melanoma according to MHC type (41).
This is the first study large enough to have adequate power to assess the immunomodulatory effects of cytokines on antigen-specific immune responses and antitumor responses of patients with refractory advanced metastatic melanoma. In this study neither IFNα nor GM-CSF significantly augmented the immunologic response to peptide vaccination of patients with active measurable metastatic melanoma. This may be explained by counter-regulatory mechanisms that may be triggered by the systemic administration of immunomodulatory cytokines. Alternatively, and given the effect of IFNα and GM-CSF upon tumor infiltrating immune cells (5) (8), it may be that the most relevant assessment of tumor immunity and vaccination would have focused upon tumor-infiltrating rather than peripheral blood antigen-specific lymphocyte responses.
The most promising finding that emerges from this study is that patients with immune response to at least one of the vaccine peptides lived longer than patients without immune response, as has previously been noted in smaller vaccine studies (38) and is remarkable for a number of reasons. First, it suggests that therapies that augment host immune response may improve the outcome of metastatic melanoma. Second, this finding suggests that immune response to vaccination may be used as a marker of improved prognosis in patients with metastatic melanoma. Our results concord well with the recent results of a separate adjuvant study upon the prognostic significance of autoimmunity in patients with resected high-risk melanoma who receive high-dose interferon—and suggest that autoimmunity is an intermediate marker of benefit from HDI through the induction of a more specific immunity to lineage and other antigens of melanoma (9). Third, understanding the molecular mechanisms regulating the development of antigen-specific immune response to vaccination may lead to further improvement of treatment for metastatic melanoma.
In summary, this large multicenter randomized phase II study of vaccination with lineage peptide antigens of melanoma administered in conjunction with two different immunomodulatory cytokines has shown that large studies with complex intermediate immunologic endpoints are feasible in the cooperative groups, using a central reference immunological monitoring laboratory. Neither of the cytokines, tested in this study was capable of significantly augmenting the immunologic or therapeutic response to vaccination. However, immune response to vaccination was associated with improved OS. This provides support for the pursuit of these same peptide vaccines in the adjuvant setting, which has been accomplished in the E4697 intergroup trial that enrolled 815 patients to rigorously test the benefit of vaccination vs. placebo using the exact same peptides and schedule tested in E1696. A better understanding of the mechanisms that regulate immune responses to melanoma vaccines is necessary before larger phase III randomized vaccine trials are conducted.
Statement of Clinical Relevance
The results of the Eastern Cooperative Oncology Group multi-epitope peptide vaccine trial E1696 trial reported in this manuscript have translational implications for the future therapy of melanoma in several respects: the results demonstrate the feasibility and immunological efficacy of multi-epitope peptide vaccination, providing the foundation for adjuvant studies of this multi-epitope peptide vaccine that are ongoing in the intergroup. In summary, the results (1) demonstrate the feasibility of vaccination with rigorous immunological assessments in the national cooperative groups, coupled with either GM-CSF or interferon alfa-2b systemic therapy tested in a factorial 2 × 2 design. This study of 120 subjects who had failed prior therapy demonstrates (2) that multi-epitope peptide vaccination induces immune response in a significant fraction of patients determined using complex ELISPOT immunological assays performed in a central reference laboratory. The modulation of immune responses assessed by ELISPOT assays of T cell IFN gamma production is correlated with improved overall survival. Finally this study (3) provides the foundation in advanced metastatic melanoma for multi-epitope peptide vaccination that has now been evaluated using a placebo-controlled design testing GM-CSF and/or peptide vaccination in 825 patients with operable stage III–IV melanoma (Intergroup E4697).
Acknowledgments
Supported by NIH Grants CA23318, CA66636, CA21115, CA39229 and research awards to ECOG from Schering Plough and Immunex Corporations.
Bibliography
- 1.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood JM, Manola J, Ibrahim J, et al. A pooled analysis of Eastern Cooperative Oncology Group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–1677. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 3.Spitler LE, Grossbard ML, Ernstoff MS, et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor (GM-CSF) J Clin Oncol. 2000;18:1614–1621. doi: 10.1200/JCO.2000.18.8.1614. [DOI] [PubMed] [Google Scholar]
- 4.Lawson D, Kirkwood JM. Granulocyte-macrophage colony-stimulating factor: another cytokine with adjuvant therapeutic benefit in melanoma? J Clin Oncol. 2000;18:1603–1605. doi: 10.1200/JCO.2000.18.8.1603. [DOI] [PubMed] [Google Scholar]
- 5.Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant Treatment of Regional Stage IIIB Melanoma with High-Dose Interferon Alfa-2b Induces Tumor Regression via an Immunomodulatory Mechanism. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 6.Panelli MC, Wang E, Phan G, et al. Gene-expression profiling of the response of peripheral blood mononuclear cells and melanoma metastases to systemic IL-2 administration. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0035. 0035.1–0035.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vuylsteke RJCLM, Molenkamp BG, Gietema HA, et al. Local Administration of Granulocyte/Macrophage Colony-stimulating Factor Increases the Number and Activation State of Dendritic Cells in the Sentinel Lymph Node of Early-Stage Melanoma. Cancer Res. 2004;64:8456–8460. doi: 10.1158/0008-5472.CAN-03-3251. [DOI] [PubMed] [Google Scholar]
- 8.Soiffer R, Hodi FS, Haluska F, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol. 2003;21:3343–3350. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Gogas H, Ioannovich J, Dafni U, et al. Prognostic Significance of Autoimmunity during Treatment of Melanoma with Interferon. NEJM. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 10.Atkins M, Mier JW, Parkinson DR, et al. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. NEJM. 1988;318:1557–1563. doi: 10.1056/NEJM198806163182401. [DOI] [PubMed] [Google Scholar]
- 11.Luiten RM, Kueter EWM, Mooi W, et al. Immunogenicity, Including Vitiligo, and Feasibility of Vaccination With Autologous GM-CSF-Transduced Tumor Cells in Metastatic Melanoma Patients. J Clin Oncol. 2005;23:8978–8991. doi: 10.1200/JCO.2005.01.6816. [DOI] [PubMed] [Google Scholar]
- 12.Astsaturov I, Petrella T, Bagriacik EU, et al. Amplication of virus-induced antimelanoma t-cell reactivity by high-dose interferon-α2b: implications for cancer vaccines. Clin Cancer Res. 2003;9:4347–4355. [PubMed] [Google Scholar]
- 13.Chianese-Bullock KA, Pressley J, Garbee C, et al. MAGE-A1-, MAGE-A10-, and gp100-Derived Peptides Are Immunogenic When Combined with Granulocyte-Macrophage Colony Stimulating Factor and Montanide ISA-51 Adjuvant and Administered as Part of a Multipeptide Vaccine for Melanoma. J Immunol. 2005;174:3080–3086. doi: 10.4049/jimmunol.174.5.3080. [DOI] [PubMed] [Google Scholar]
- 14.Zarour H, Kirkwood JM. Melanoma vaccines: Early progress and future promises. Semin Cutan Med Surg. 2003;22:68–75. doi: 10.1053/sder.2003.50006. [DOI] [PubMed] [Google Scholar]
- 15.Slingluff CL, Petroni GR, Yamshchikov GV, et al. Immunologic and Clinical Outcomes of Vaccination With a Multiepitope Melanoma Peptide Vaccine Plus Low-Dose Interleukin-2 Administered Either Concurrently or on a Delayed Schedule. J Clin Oncol. 2004;22:4474–4485. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- 16.Slingluff CL, Jr., Petroni GR, Yamshchikov GV, et al. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulting factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Bade E, Kuniyoski C, et al. Phase I trial of a MART-1 peptide vaccine with incomplete freund's adjuvant for resected high-risk melanoma. Clin Cancer Res. 1999;5:2756–2765. [PubMed] [Google Scholar]
- 18.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis ID, Chen W, Jackson H, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4+ and CD8+ T cell responses in humans. Proc Nat Acad Sci U.S.A. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traversari C, van der Bruggen P, Leuscher IF, et al. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992;176:1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfel T, van Pel A, Brichard V, et al. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolyticT lymphocytes. Eur J Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 23.Cox AL, Skipper J, Chen Y, et al. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 24.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami Y, Eliyahu S, Delgado CH, et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coulie PG, Brichard V, van Pel A, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami Y, Eliyahu S, Sakaguchi K, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castelli C, Storkus WJ, Maeurer MJ, et al. Mass spectrometric identification of a naturally processed melanoma peptide recognized by CD8+ cytotoxic T lymphocytes. J Exp Med. 1995;181:363–368. doi: 10.1084/jem.181.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkwood JM, Bender C, Agarwala SS, et al. Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J Clin Oncol. 2002;20:3703–3718. doi: 10.1200/JCO.2002.03.052. [DOI] [PubMed] [Google Scholar]
- 30.Yamshchikov G, Barnd DL, Eastham S, et al. Evaluation of peptide vaccine immunogenicity in draining lymph nodes and peripheral blood of melanoma patients. Int J Cancer. 2001;92:703–711. doi: 10.1002/1097-0215(20010601)92:5<703::aid-ijc1250>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Sondak VK, Liu P-Y, Tuthill RJ, et al. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: overall results of a randomized trial of the Southwest Oncology Group. J Clin Oncol. 2002;20:2058–2066. doi: 10.1200/JCO.2002.08.071. [DOI] [PubMed] [Google Scholar]
- 32.Hersey P, Coates AS, McCarthy WH, et al. Adjuvant Immunotherapy of Patients With High-Risk Melanoma Using Vaccinia Viral Lysates of Melanoma: Results of a Randomized Trial. J Clin Oncol. 2002;20:4181–4190. doi: 10.1200/JCO.2002.12.094. [DOI] [PubMed] [Google Scholar]
- 33.Hsueh EC, Morton DL. Antigen-based immunotherapy of melanoma: Canvaxin therapeutic polyvalent cancer vaccine. Semin Cancer Biol. 2003;13:401–407. doi: 10.1016/j.semcancer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Kirkwood JM, Ibrahim J, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: Results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 35.Pass HA, Schwarz SL, Wunderlich JR, et al. Immunization of patients with melanoma peptide vaccines: immunologic assessment using the ELISPOT assay. Cancer J Sci Am. 1998;4:316–323. [PubMed] [Google Scholar]
- 36.Markovic SN, Suman VJ, Ingle JN, et al. Peptide vaccination of patients with metastatic melanoma: improved clinical outcome in patients demonstrating effective immunization. Am J Clin Oncol. 2006;29:352–360. doi: 10.1097/01.coc.0000217877.78473.a4. [DOI] [PubMed] [Google Scholar]
- 37.Lotem M, Zhao Y, Riley J, et al. Presentation of tumor antigens by dendritic cells genetically modified with viral and nonviral vectors. J Immunother. 2006;29:616–627. doi: 10.1097/01.cji.0000211312.36363.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufmann R, Spieth K, Leiter U, et al. Temozolomide in Combination With Interferon-Alfa Versus Temozolomide Alone in Patients With Advanced Metastatic Melanoma: A Randomized, Phase III, Multicenter Study from the Dermatologic Cooperative Oncology Group. J Clin Oncol. 2005;23:9001–9007. doi: 10.1200/JCO.2005.01.1551. [DOI] [PubMed] [Google Scholar]
- 39.Keiholz U, Punt CJA, Gore M, et al. Dacarbazine, Cisplatin, and Interferon-Alfa-2b With or Without Interleukin-2 in Metastatic Melanoma: A Randomized Phase III Trial (18951) of the European Organization for Research and Treatment of Cancer Melanoma Group. J Clin Oncol. 2005;23:6747–6755. doi: 10.1200/JCO.2005.03.202. [DOI] [PubMed] [Google Scholar]
- 40.Kirkwood JM, Richards T, Zarour HM, et al. Immunomodulatory effects of high-dose and low-dose interferon alpha2b in patients with high-risk resected melanoma: the E2690 laboratory corollary of intergroup adjuvant trial E1690. Cancer. 2002;95:1101–1112. doi: 10.1002/cncr.10775. [DOI] [PubMed] [Google Scholar]
- 41.Peterson AC, Gajewski TF. Immunization with Melan-A peptide-pulsed peripheral blood mononucvlear cells plus recombinant human interleukin-12 induced clinidal activity and T-cell responses in advanced melanoma. J Clin Oncol. 2003;21:2342–2348. doi: 10.1200/JCO.2003.12.144. [DOI] [PubMed] [Google Scholar]