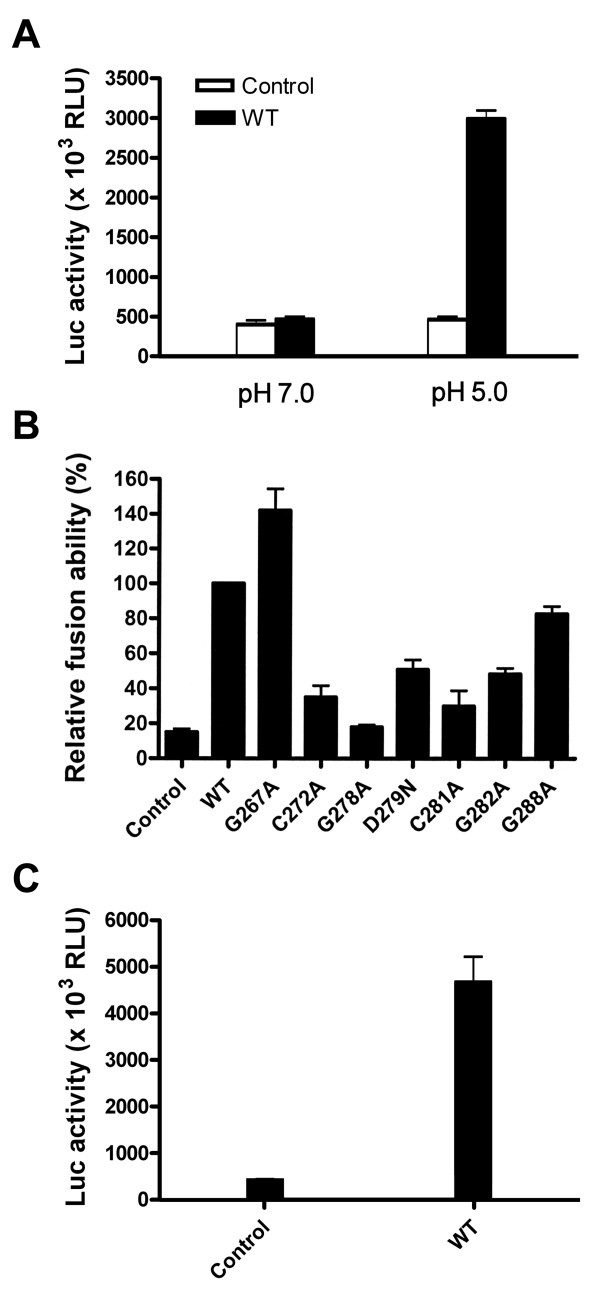
Figure 3.
Membrane fusion ability of mutant proteins. (A) Fusion ability of WT E1 and E2 at neutral and acidic pHs. 293T cells were cotransfected with pCAG-T7Pol and pcDNA3 (marked as the control) or the WT E1E2 plasmid as indicated, and Huh 7 cells were transfected with pT7EMCVLuc. Two days post-transfection, cells were detached, resuspended, and added to transfected Huh 7 cells. Five h after coculture, cells were treated with PBS at pH 7.0 and pH 5.0, respectively, and 37°C for 2 min, and then incubated for an additional 5 h. Cells were lysed and assayed for firefly luciferase activity. The mean values of luciferase activities from three separate analyses with standard deviation are shown. (B and C) The membrane fusion ability of mutant proteins. 293T cells were cotransfected with pCAG-T7Pol and pcDNA3 (marked as the control), WT, or mutant E1E2 plasmids, and the membrane fusion abilities of WT and mutant proteins were assessed at pH 5.0 as described in (A). The luciferase activity was measured, and the relative fusion abilities of the mutants were expressed as a percentage of that of the WT E1E2 (B). The actual values of luciferase activities of the control and WT samples shown in (B) are depicted in (C). All the diagrams represent the results from three independent experiments (mean ± standard deviation), each of which was performed with triplicate samples.