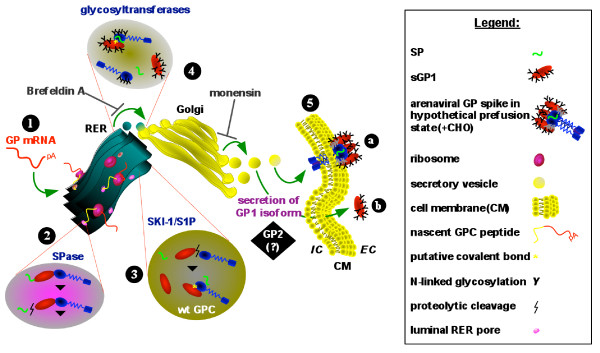
Figure 6.
Schematic representation of arenaviral glycoprotein expression pathway and secretory forms. Arenaviral glycoprotein (GP) mRNA is transcribed from plasmid DNA in the host cell (1), and is directed to the rough endoplasmic reticulum (RER) for translation by associated ribosomes. The nascent polyprotein is directed to the RER lumen by the unique arenaviral SP, where SPases cleave the SP from the GP polyprotein precursor (2). In the RER the GP precursor is cleaved by SKI-1/S1P to yield GP1 and GP2 subunits (3). Wild type GP is possibly comprised of covalently linked, or non-covalently associated GP1 and GP2 subunits, and associated SP (3). Glycosylation of GP1 and GP2 is mediated by glycosyltransferases in the RER or in the Golgi cisternae (4). Membrane bound vesicles transport proteins to the cis face of the Golgi where final processing and assembly of the GP complex occurs. Fully assembled and glycosylated GP complex or subunits are packaged in exocytic vesicles which emerge from the trans face of the Golgi and are directed toward the plasma membrane. Glycoprotein complex is anchored to the cell membrane via the transmembrane domain of GP2 (5a). A complexed heterodimeric GP1-GP2 is displayed in 5a. Extracellular GP1 originating from GPC expression is a secreted glycoprotein isoform (5b). The positions of BFA and monensin inhibitory action along the secretory pathway are indicated. The role of LASV GP2 in the secretory pathway of GP1 was not elucidated in these studies (black box). A graphic representation of each relevant component in the outlined pathway is shown in the legend box.