Figure 1.
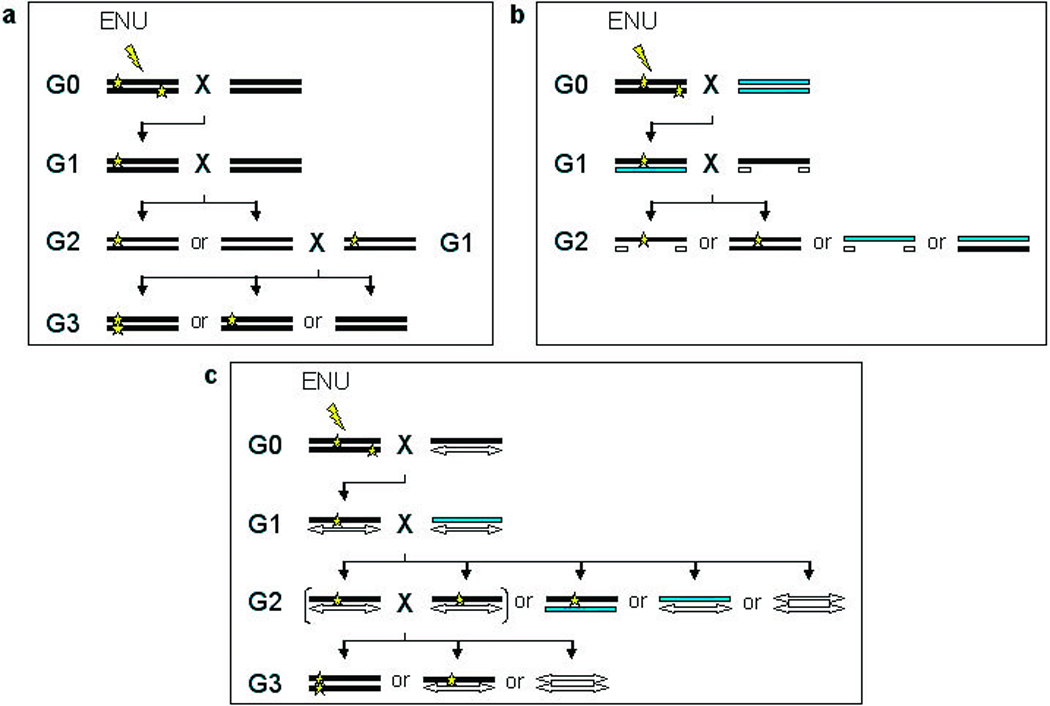
Methods for ENU mutagenesis. a, In a basic ENU mutagenesis screen, a male G0 mouse is treated with ENU and mated to a wild type female. The G1 progeny can be screened for dominant mutations. To screen for recessive mutations, the G1 carrier is mated to a wild type mouse to produce potential carriers in the G2 generation. The G2 mice can then be backcrossed to the original G1 parent to generate homozygotes in the G3 [3, 4, 6]. b, To more rapidly screen for recessive mutations within a particular region, a deletion chromosome may be used. The mutagenized G0 male is bred to a wild type female. The resulting G1 potential carriers can then be bred to mice carrying a deletion, and the G2 progeny can be screened for recessive mutations within the deleted region a generation earlier than the basic screen (a). The use of dominant markers, such as coat color, indicated by the grey and white chromosomes, can facilitate identification of potential mutants to be screened [3, 6, 9]. c, Additionally, chromosomes with a large inversion and a coat color marker, called balancer chromosomes, are useful for recovering and maintaining recessive mutations. The mutagenized G0 mouse is bred with a female carrying a balancer chromosome (white double-headed arrow). The resulting G1 progeny carrying the balancer are then bred to another mouse carrying the balancer chromosome and an alternative coat color marker (gray bar). The G2 progeny carrying both the balancer chromosome and the mutagenized chromosome can be identified by coat color and intercrossed, producing only carriers and potential mutants for screening, which are distinguishable by coat color. Progeny receiving two balancer chromosomes may not be viable [3, 4, 6, 10].
